Bone quality relies on hyaluronan synthesis - Insights from mice with complete knockout of hyaluronan synthase expression
- PMID: 39736893
- PMCID: PMC11683482
- DOI: 10.1016/j.mbplus.2024.100163
Bone quality relies on hyaluronan synthesis - Insights from mice with complete knockout of hyaluronan synthase expression
Abstract
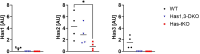
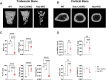
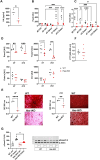
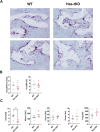
Bone consists of a complex mineralised matrix that is maintained by a controlled equilibrium of synthesis and resorption by different cell types. Hyaluronan (HA) is an important glycosaminoglycan in many tissues including bone. Previously, the importance of HA synthesis for bone development during embryogenesis has been shown. We therefore investigated whether HA synthesis is involved in adult bone turnover and whether abrogation of HA synthesis in adult mice would alter bone quality. To achieve complete abrogation of HA synthesis in adult mice, we generated a novel Has-total knockout (Has-tKO) mouse model in which a constitutive knockout of Has1 and Has3 was combined with an inducible, Ubc-Cre-driven Has2 knockout. By comparing bone tissue from wild-type, Has1,3 double knockout and Has-tKO mice, we demonstrate that Has2-derived HA mainly contributes to the HA content in bone. Furthermore, Has-tKO mice show a significant decrease of bone integrity in trabecular and cortical bone, as shown by µ-CT analysis. These effects are detectable as early as five weeks after induced Has2 deletion, irrespective of sex and progress with age. Mesenchymal stem cells (MSC) during osteogenic differentiation in vitro showed that Has2 expression is increased while Has3 expression is decreased during differentiation. Furthermore, the complete abrogation of HA synthesis results in significantly reduced osteogenic differentiation as indicated by reduced marker gene expression (Runx-2, Tnalp, Osterix) as well as alizarin red staining. RNAseq analysis revealed that MSC from Has-tKO are characterised by decreased expression of genes annotated for bone and organ development, whereas expression of genes associated with chemokine related interactions and cytokine signalling is increased. Taken together, we present a novel mouse model with complete deletion of HA synthases in adult mice which has the potential to study HA function in different organs and during age-related HA reduction. With respect to bone, HA synthesis is important for maintaining bone integrity, presumably based on the strong effect of HA on osteogenic differentiation.
Keywords: Bone; Hyaluronan synthase; Osteogenic differentiation.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Camenisch T.D., Spicer A.P., Brehm-Gibson T., Biesterfeldt J., Augustine M.L., Calabro A., Kubalak S., Klewer S.E., McDonald J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

