Blood component therapy for dry eye disease: a systematic review and network meta-analysis
- PMID: 39736981
- PMCID: PMC11683103
- DOI: 10.3389/fmed.2024.1500160
Blood component therapy for dry eye disease: a systematic review and network meta-analysis
Abstract
Objective: Blood component therapy has shown promising potential as an emerging treatment for dry eye disease; however, it remains unclear which specific blood component is the most effective. This study aims to compare the efficacy of different blood components in the treatment of dry eye disease through a network meta-analysis, with the goal of providing the latest and most reliable evidence for clinical practice.
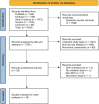
Methods: We conducted a systematic search of the PubMed, Web of Science, Cochrane, Embase, and Scopus databases, with the search concluding on June 1, 2024. Two independent researchers performed literature screening, data extraction, and quality assessment.
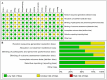
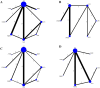
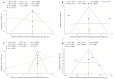
Results: A total of 16 randomized controlled trials (RCTs) involving 898 patients with dry eye disease were included. Six different blood components were utilized in treating dry eye disease, with platelet-rich plasma (PRP) being the most widely used. The results of the network meta-analysis indicated that platelet-rich plasma eye drops (PRPD) significantly outperformed artificial tears (AT) in improving the corneal fluorescein staining score (CFSS), while autologous serum (ALS) and umbilical cord serum (UCS) also demonstrated significantly better effects than AT in enhancing tear break-up time (TBUT). Additionally, ALS, PRP injection (PRPI), and PRPD showed significantly superior outcomes compared to AT in improving the ocular surface disease index (OSDI). However, no statistically significant differences were found among the various treatment modalities regarding their effects on Schirmer's I value, CFSS, TBUT, and OSDI. SUCRA analysis predicted that UCS was the most effective in improving Schirmer's I value and TBUT, while PRP excelled in enhancing CFSS and OSDI. Limitations such as publication bias and issues related to randomization, allocation concealment, and blinding may affect the reliability of the current findings.
Conclusion: Blood component therapy can significantly improve the pathological damage and ocular surface health in patients with dry eye disease. For those with aqueous-deficient dry eye, UCS may represent the optimal treatment option. In contrast, for patients with more severe corneal epithelial damage, PRP may offer a more effective therapeutic approach.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, CRD42024534091.
Keywords: blood component therapy; dry eye disease; network meta-analysis; randomized controlled trial; systematic review.
Copyright © 2024 Zhang, Li, Ge and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Therapeutic Effects of Umbilical Cord Serum for Dry Eye Disease: A Systemic Review and Meta-Analysis.Ophthalmic Res. 2025;68(1):71-83. doi: 10.1159/000542731. Epub 2024 Dec 2. Ophthalmic Res. 2025. PMID: 39622221 Free PMC article.
-
How effective and safe are punctal plugs in treating dry eye disease? A systematic review and meta-analysis.Cont Lens Anterior Eye. 2025 May 19:102438. doi: 10.1016/j.clae.2025.102438. Online ahead of print. Cont Lens Anterior Eye. 2025. PMID: 40393913 Review.
-
Topical cyclosporine A therapy for dry eye syndrome.Cochrane Database Syst Rev. 2019 Sep 13;9(9):CD010051. doi: 10.1002/14651858.CD010051.pub2. Cochrane Database Syst Rev. 2019. PMID: 31517988 Free PMC article.
-
Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Ophthalmic Res. 2020;63(5):443-451. doi: 10.1159/000505630. Epub 2019 Dec 30. Ophthalmic Res. 2020. PMID: 31884498
-
Comparison of novel lipid-based eye drops with aqueous eye drops for dry eye: a multicenter, randomized controlled trial.Clin Ophthalmol. 2015 Apr 15;9:657-64. doi: 10.2147/OPTH.S74849. eCollection 2015. Clin Ophthalmol. 2015. PMID: 25931806 Free PMC article.
Cited by
-
Platelet-Rich Plasma for the Treatment of Ocular Surface Disease in Animals: A Systematic Review.Vet Med Int. 2025 Jun 23;2025:9921619. doi: 10.1155/vmi/9921619. eCollection 2025. Vet Med Int. 2025. PMID: 40590020 Free PMC article. Review.
-
Real-World Outcomes of Allogeneic Immunosafe Plasma Rich in Growth Factors Eye Drops for Refractory Ocular Surface Diseases: A Prospective Observational Study.Ophthalmol Ther. 2025 Sep;14(9):2253-2281. doi: 10.1007/s40123-025-01211-1. Epub 2025 Jul 25. Ophthalmol Ther. 2025. PMID: 40715787 Free PMC article.
-
Biological topicals in ocular surface disorders.Indian J Ophthalmol. 2025 Apr 1;73(4):496-507. doi: 10.4103/IJO.IJO_482_25. Epub 2025 Mar 27. Indian J Ophthalmol. 2025. PMID: 40146137 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

