Ultrastructural morphology of second and third-stage larvae of Toxocara cati inside paratenic host tissue
- PMID: 39737013
- PMCID: PMC11682779
- DOI: 10.5455/OVJ.2024.v14.i11.27
Ultrastructural morphology of second and third-stage larvae of Toxocara cati inside paratenic host tissue
Abstract
Background: Toxocara cati is a known cause of a zoonotic infectious illness called toxocariasis. Parathenic hosts are important as they can transmit larvae 2 (L2) through direct transmission. Scanning electron microscope (SEM) techniques are needed to provide a three-dimensional image of each stage of T. cati larvae.
Aim: The aim of this study was to determine the morphology of L2 and L3 T. cati in parathenic host tissue for etiological diagnosis using SEM.
Methods: Mice were used as suitable paratenic hosts for this experiment. A total of 786 embryonated eggs (16 eggs/gram body weight) containing L2 were inoculated into pregnant mice at day 11-13 of its gestation period. After delivery, L2 was transmitted to the off-spring. After 14 days, L2 was collected from mice, and L3 was collected from its off-spring. Data were analyzed descriptively based on ultrastructure examination using SEM.
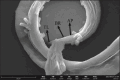
Results: SEM examination results indicate that the size of L2 is smaller than L3. Results also showed differences between L2 and L3 based on middle and posterior observations. In the middle of the larval body, the number of L2 body rings was observed to be narrower and more than that of L3. In addition, the distance between L2 body rings was much larger than that of L3. Posteriorly, the tail tip of L3 was more curved than L2.
Conclusion: Ultrastructural examination using SEM has the ability to show differences in L2 and L3 body rings of T. cati by observing the middle and posterior parts of its larvae.
Keywords: Larvae; Parathenic host; Scanning electron microscopy; Toxocara cati; Zoonosis.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





References
-
- Abou-El-Naga, I.F., El-Nassery, S.M.F. and Sharaf, I.A. 2022. Immunochemical studies of Toxocara canis proteases. Trop. Biomed. 39(3), 315–320. - PubMed
-
- Bakhshani, A., Khodaverdi, M. and Borji, H. 2020. Distribution of Toxocara cati larvae in experimentally infected BALB/c mice. Vet. Parasitol. 285(1), 109220. - PubMed
-
- Bowman, D.D., Oaks, J.A. and Grieve, R.B. 1993. Ultrastructure of the infective stage larva of Toxocara canis (nematoda: ascaridoidea). Proc. Helminthol. Soc. Wash. 60(2), 183–204.
-
- Candra, D.A., Lastuti, N.D.R., Hidayati, N., Kusnoto, Hastutiek, P. and Bijanti, R. 2020. Differential counting value and determining of leukocytes in chicken after infected with L2 Toxocara cati. J. Parasite Sci. 4(1), 11–16.
MeSH terms
LinkOut - more resources
Full Text Sources
