Comparison of intravitreal anti-VEGF agents and oral carbonic anhydrase inhibitors in the treatment of cystoid macular edema secondary to retinitis pigmentosa
- PMID: 39737070
- PMCID: PMC11683215
- DOI: 10.3389/fphar.2024.1477889
Comparison of intravitreal anti-VEGF agents and oral carbonic anhydrase inhibitors in the treatment of cystoid macular edema secondary to retinitis pigmentosa
Abstract
Purpose: To compare the efficacy of intravitreal antivascular endothelial growth factor (anti-VEGF) agents with oral carbonic anhydrase inhibitors (CAIs) in treating cystoid macular edema (CME) secondary to retinitis pigmentosa (RP).
Methods: This retrospective study analyzed 98 patients (98 eyes) with RP-CME: 47 (48.0%) received intravitreal anti-VEGF agents (Ranibizumab or Bevacizumab) and 51 (52.0%) were treated with oral CAIs (methazolamide 50 mg/day or acetazolamide 500 mg/day). Medical records were reviewed to assess best-corrected visual acuity (BCVA), central macular thickness (CMT), and intraocular pressure (IOP) at baseline and at 1, 3, 6, and 12 months post-treatment using Generalized Estimation Equations (GEE). Adverse events and risk factors influencing visual prognosis were also evaluated.
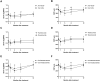
Results: Both groups showed significant improvement in BCVA and reduction in CMT at 1 and 3 months post-treatment compared to baseline (all p < 0.001). In the oral CAIs group, these improvements persisted until 6 months. However, by 12 months, neither group exhibited significant improvements in BCVA or CMT compared to baseline (all p > 0.05). Intragroup comparisons revealed that the oral CAIs group had significantly better BCVA and CMT improvements at 3 and 6 months than intravitreal anti-VEGF group (p < 0.001 for BCVA at 3 months, p = 0.003 for BCVA at 6 months; all p < 0.001 for CMT at both 3 and 6 months). No significant differences were found between the two groups in BCVA and CMT at 12 months or in IOP at any time point (all p > 0.05). Subgroup analysis indicated that oral acetazolamide was more effective than methazolamide in reducing CMT and improving BCVA at 3 and 6 months (p = 0.005 for BCVA at 3 months, p = 0.015 for BCVA at 6 months; p = 0.037 for CMT at 3 months, p < 0.001 for CMT at 6 months). There were no significant differences in outcomes between intravitreal Ranibizumab and Bevacizumab (all p > 0.05). Correlation analysis showed that worse BCVA at 12 months was associated with older age (r = 0.202, p = 0.046), higher baseline CMT (r = 0.353, p < 0.001), poorer baseline BCVA (r = 0.579, p < 0.001), but showed no correlation with genotype. Adverse effects from oral CAIs included tingling sensation (3.9%), altered taste (9.8%), and gastrointestinal upset (7.8%). The Ranibizumab group required an average of 3.7 ± 0.8 treatments, and the Bevacizumab group required an average of 3.8 ± 0.5 treatments over the course of 1 year without experiencing severe adverse effects.
Conclusion: Both intravitreal anti-VEGF agents and oral CAIs effectively improved CMT and BCVA in RP-CME patients within the first 3 months of treatment. However, oral CAIs demonstrated superior anatomic and functional improvements at 6 months. Poorer BCVA prognosis was associated with older age, higher baseline CMT, poorer baseline visual acuity. Larger, randomized clinical trials with extended follow-up periods are needed to confirm these findings.
Keywords: anti-vegf; carbonic anhydrase inhibitors; clinical efficacy; cystoid macular edema; retinitis pigmentosa.
Copyright © 2024 Liang, Wu, Chen, Feng, Hei, Diao, Ji, Zheng, Zou, Fang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

