Broccoli (Brassica oleracea var. italica) leaves exhibit significant antidiabetic potential in alloxan-induced diabetic rats: the putative role of ABC vacuolar transporter for accumulation of Quercetin and Kaempferol
- PMID: 39737071
- PMCID: PMC11683327
- DOI: 10.3389/fphar.2024.1421131
Broccoli (Brassica oleracea var. italica) leaves exhibit significant antidiabetic potential in alloxan-induced diabetic rats: the putative role of ABC vacuolar transporter for accumulation of Quercetin and Kaempferol
Abstract
Background: The global prevalence of diabetes among adults over 18 years of age is expected to increase from 10.5% to 12.2% (between 2021 and 2045). Plants can be a cost-effective source of flavonoids like quercetin and kaempferol with anti-diabetic properties.
Methodology: We aimed to assess the antidiabetic potential of leaves of Brassica oleracea cvs. Green Sprout and Marathon. Further, flavonoid contents were measured in broccoli leaves grown under light and dark conditions. The methanolic extracts of Green Sprout (GSL-M) and Marathon (ML-M) were first evaluated in vitro for their α-amylase and α-glucosidase inhibitory potential and then for antidiabetic activity in vivo in alloxan-induced diabetic rat models.
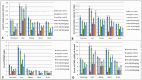
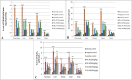
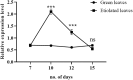
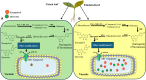
Results: Treatment with plant extracts promoted the reduced glutathione (GSH) content and CAT, POD, and SOD activities in the pancreas, liver, kidney, heart, and brain of diabetic rats, whereas lowered lipid peroxidation, H2O2, and nitrite concentrations. The histopathological studies revealed the protective effect of plant extracts at high dose (300 mg/kg), which could be due to broccoli's rich content of chlorogenic acid, quercetin, and kaempferol. Strikingly, etiolated leaves of broccoli manifested higher levels of quercetin and kaempferol than green ones. The putative role of an ABC transporter in the accumulation of quercetin and kaempferol in etiolated leaves was observed as evaluated by qRT-PCR and in silico analyses.
Conclusion: In conclusion, the present study shows a strong link between the antidiabetic potential of broccoli due to the presence of chlorogenic acid, quercetin, and kaempferol and the role of an ABC transporter in their accumulation within the vacuole.
Keywords: ABC transporter; Brassica oleracea; alloxan; antidiabetic; antioxidant enzymes; flavonoids; histopathology; lipid peroxidation.
Copyright © 2024 Latif, Sameeullah, Abbasi, Masood, Demiral Sert, Aslam, Pekdemir, Imren, Çiftçi, Saba, Malik, Ijaz, Batool, Mirza and Waheed.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



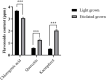

References
-
- Agbon A. N., Ingbian S. D., Dahiru A. U. (2014). Preliminary histological and histochemical studies on the neuroprotective effect of aqueous fruit extract of Phoenix dactylifera L. (Date Palm) on atesunate - induced cerebellar damage in wistar rats. Sub-Saharan Afr. J. Med 1, 204. 10.4103/2384-5147.144744 - DOI
-
- Aslam N., Sameeullah M., Yildirim M., Baloglu M. C., Yucesan B., Lössl A. G., et al. (2022). Isolation of the 3β-HSD promoter from Digitalis ferruginea subsp. ferruginea and its functional characterization in Arabidopsis thaliana . Mol. Biol. Rep. 49, 7173–7183. 10.1007/s11033-022-07634-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

