Precision medicine for patients with salivary gland neoplasms: Determining the feasibility of implementing a next-generation sequencing-based RNA assay in a hospital laboratory
- PMID: 39737124
- PMCID: PMC11683391
- DOI: 10.25259/Cytojournal_152_2024
Precision medicine for patients with salivary gland neoplasms: Determining the feasibility of implementing a next-generation sequencing-based RNA assay in a hospital laboratory
Abstract
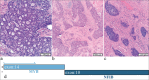
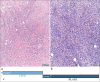
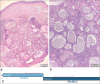
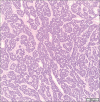
Objective: Diagnosing neoplasms of the salivary gland is challenging, as morphologic features of these tumors are complex, and well-defined diagnostic categories have overlapping features. Many salivary gland neoplasms are associated with recurrent genetic alterations. The utilization of RNA-based targeted next-generation sequencing (NGS) panels for the detection of cancer-driving translocations and mutations is emerging in the clinical laboratory. Our objective was to conduct a proof-of-concept study to show that in-house molecular testing of salivary gland tumors can enhance patient care by supporting morphologic diagnoses, thereby improving therapeutic strategies such as surgical options and targeted therapies.
Material and methods: Residual formalin-fixed paraffin-embedded salivary gland neoplasm specimens from a cohort of 17 patients were analyzed with the Archer FusionPlex Pan Solid Tumor v2 panel by NGS on an Illumina NextSeq550 platform.
Results: We identified structural gene rearrangements and single nucleotide variants in our patient samples that have both diagnostic and treatment-related significance. These alterations included PLAG1, MAML, and MYB fusions and BRAF, CTNNB1, NRAS, and PIK3CA mutations.
Conclusion: Our RNA-based NGS assay successfully detected known gene translocations and mutations associated with salivary gland neoplasms. The genetic alterations detected in these tumors demonstrated potential diagnostic, prognostic, and therapeutic value. We suggest that incorporating in-house ancillary molecular testing could greatly enhance the accuracy of salivary gland fine needle aspiration cytology and small biopsies, thereby better guiding surgical decisions and the use of targeted therapies.
Keywords: RNA sequencing; fine-needle aspiration; next-generation sequencing; precision medicine; salivary gland.
© 2024 The Author(s). Published by Scientific Scholar.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Skalova A, Hyrcza MD. Proceedings of the North American Society of Head and Neck Pathology Companion Meeting, New Orleans, LA, March 12, 2023: Classification of salivary gland tumors: Remaining controversial issues? Head Neck Pathol. 2023;17:285–91. doi: 10.1007/s12105-023-01541-1. - DOI - PMC - PubMed
-
- Moutasim KA, Thomas GJ. Salivary gland tumours: Update on molecular diagnostics. Diagn Histopathol. 2020;26:159–64. doi: 10.1016/j.mpdhp.2020.01.002. - DOI
-
- Christensen PA, Subedi S, Pepper K, Hendrickson HL, Li Z, Thomas JS, et al. Development and validation of Houston Methodist Variant Viewer version 3: Updates to our application for interpretation of next-generation sequencing data. JAMIA Open. 2020;3:299–305. doi: 10.1093/jamiaopen/ooaa004. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
