Surgical navigation for targeted retroperitoneal lymph-node removal: a randomised, controlled, phase 3 trial
- PMID: 39737148
- PMCID: PMC11683954
- DOI: 10.1016/j.eclinm.2024.102754
Surgical navigation for targeted retroperitoneal lymph-node removal: a randomised, controlled, phase 3 trial
Abstract
Background: Metastatic retroperitoneal lymph node dissection (LND) for nodal recurrence is applied for a variety of cancers, such as urological, gynaecological and rectal cancer. Precise localisation and resection of these lymph nodes (LNs) during surgery can be challenging, especially after previous radiotherapy or surgery. The objective of this study was to assess the added value of surgical navigation for targeted LND in the retroperitoneum.
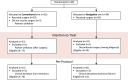
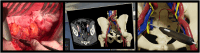
Methods: We performed an open-label randomised, controlled, phase 3 trial at the Netherlands Cancer Institute, Amsterdam. Eligible participants were over 18 years of age, scheduled for targeted retroperitoneal LND by laparotomy, with removal of one or more suspected (targeted) LN(s) as assessed by diagnostic imaging. Patients were randomised (1:1) between conventional LND and LND using surgical navigation, by means of a minimisation method stratified for tumour origin (urological, colorectal and other). For the surgical navigation, a digital 3D model of the patients' anatomy was created from diagnostic CT scans, including delineation of the targeted LN(s). The 3D model was linked to the patients' position in the operation room. Using an electromagnetic tracking system, with a sterile tracked pointer, the actual position of the pointer was shown in the 3D model, enabling the surgeon to localize the targeted LN(s). The primary outcome of the study was the percentage of successful procedures. Success was defined as no residual target LN(s) visible on postoperative CT imaging. This study was registered with ClinicalTrials.gov, NCT05867095.
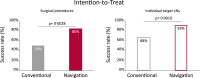
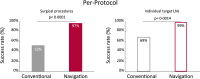
Findings: From January 2017 to December 2020, 69 participants were included in the study, 35 (51%) in the conventional arm and 34 (49%) in the navigation arm. Four patients were not evaluable and excluded from further analysis; three in the conventional arm (patients withdraw from study participation), one in the navigation arm (discontinued surgery, misclassified diagnosis). According to intention-to-treat analysis, 50% (16/32) of the surgical procedures was successful in the conventional arm, versus 85% (28/33) in the surgical navigation arm (one-tailed p = 0.0028, 90% CI: 14%-56%). Using the Clavien-Dindo classification, the overall complication rate was comparable between the conventional arm and the navigation arm. Surgeons judged the surgical navigation setup as valuable, the median preference score to use surgical navigation was 3.7 (3.3-4.0) (scale 1-5), and the median system usability score was 75 (70-85) (scale 0-100).
Interpretation: Surgical navigation allows for significantly better localisation and removal of target LN(s) in the retroperitoneum.
Funding: This research was supported by the KWF-Alpe d'HuZes (NKI 2014-6596) and by an institutional grant of The Dutch Cancer Society and of the Dutch Ministry of Health, Welfare and Sport.
Keywords: 3D model for surgical localisation; Electromagnetic tracking; Retroperitoneal lymph node dissection; Surgical navigation.
© 2024 The Authors.
Conflict of interest statement
TR is involved as CMO of a company in surgical navigation, Bcon Medical.
Figures

References
-
- Hofman M.S., Lawrentschuk N., Francis R.J., et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. - PubMed
-
- Perera M., Papa N., Roberts M., et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77:403–417. - PubMed
-
- de Barros H.A., van Oosterom M.N., Donswijk M.L., et al. Robot-assisted prostate-specific membrane antigen–radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur Urol. 2022;82:97–105. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

