Functional MRGPRX2 expression on peripheral blood-derived human mast cells increases at low seeding density and is suppressed by interleukin-9 and fetal bovine serum
- PMID: 39737168
- PMCID: PMC11683848
- DOI: 10.3389/fimmu.2024.1506034
Functional MRGPRX2 expression on peripheral blood-derived human mast cells increases at low seeding density and is suppressed by interleukin-9 and fetal bovine serum
Abstract
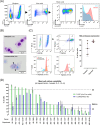
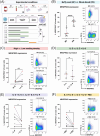
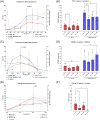
Primary human mast cells (MC) obtained through culturing of blood-derived MC progenitors are the preferred model for the ex vivo study of MRGPRX2- vs. IgE-mediated MC activation. In order to assess the impact of culture conditions on functional MRGPRX2 expression, we cultured CD34+-enriched PBMC from peripheral whole blood (PB) and buffy coat (BC) samples in MethoCult medium containing stem cell factor (SCF) and interleukin (IL)-3, modified through variations in seeding density and adding or withholding IL-6, IL-9 and fetal bovine serum (FBS). Functional expression of MRGPRX2 was assessed after 4 weeks via flow cytometry. We found similar proportions of CD34+ MC-committed progenitors in BC and PB. Higher seeding densities (≥ 1x105 cells/mL) and exposure to IL-9 and FBS suppressed functional MRGPRX2 expression at 4 weeks, while leaving MC yield largely unaffected. IL-6 had no impact on MRGPRX2 expression. MRGPRX2-expressing MC upregulated CD63 upon stimulation with polyclonal anti-IgE, substance P and compound 48/80 at 4 weeks. Ketotifen and dasatinib but not cromolyn sodium inhibited both IgE- and MRGPRX2-dependent pathways. Our results confirm the feasibility of functional MC activation studies on PB-derived MC after a short 4-week culture and highlight the impact of culture conditions on functional MRGPRX2 expression.
Keywords: MRGPRX2; blood; culture; human; interleukin-9; ketotifen; mast cell; serum.
Copyright © 2024 Ieven, Goossens, Roosens, Jonckheere, Cremer, Dilissen, Persoons, Dupont, Schrijvers, Vandenberghe, Breynaert and Bullens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Peripheral blood cultured mast cells: Phenotypic and functional outcomes of different culture protocols.J Immunol Methods. 2021 May;492:113003. doi: 10.1016/j.jim.2021.113003. Epub 2021 Feb 26. J Immunol Methods. 2021. PMID: 33647250
-
MRGPRX2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture.Exp Dermatol. 2018 Nov;27(11):1298-1303. doi: 10.1111/exd.13762. Epub 2018 Sep 3. Exp Dermatol. 2018. PMID: 30091263
-
Novel Insights on MRGPRX2-Mediated Hypersensitivity to Neuromuscular Blocking Agents And Fluoroquinolones.Front Immunol. 2021 Jul 27;12:668962. doi: 10.3389/fimmu.2021.668962. eCollection 2021. Front Immunol. 2021. PMID: 34385999 Free PMC article.
-
MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis?Exp Dermatol. 2020 Nov;29(11):1104-1111. doi: 10.1111/exd.14182. Epub 2020 Sep 17. Exp Dermatol. 2020. PMID: 32866307 Review.
-
Mast cell degranulation and bradykinin-induced angioedema - searching for the missing link.Front Immunol. 2024 May 15;15:1399459. doi: 10.3389/fimmu.2024.1399459. eCollection 2024. Front Immunol. 2024. PMID: 38812508 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

