Deciphering ferroptosis in critical care: mechanisms, consequences, and therapeutic opportunities
- PMID: 39737174
- PMCID: PMC11682965
- DOI: 10.3389/fimmu.2024.1511015
Deciphering ferroptosis in critical care: mechanisms, consequences, and therapeutic opportunities
Abstract
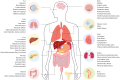
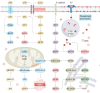
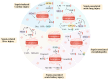
Ischemia-reperfusion injuries (IRI) across various organs and tissues, along with sepsis, significantly contribute to the progression of critical illnesses. These conditions disrupt the balance of inflammatory mediators and signaling pathways, resulting in impaired physiological functions in human tissues and organs. Ferroptosis, a distinct form of programmed cell death, plays a pivotal role in regulating tissue damage and modulating inflammatory responses, thereby influencing the onset and progression of severe illnesses. Recent studies highlight that pharmacological agents targeting ferroptosis-related proteins can effectively mitigate oxidative stress caused by IRI in multiple organs, alleviating associated symptoms. This manuscript delves into the mechanisms and signaling pathways underlying ferroptosis, its role in critical illnesses, and its therapeutic potential in mitigating disease progression. We aim to offer a novel perspective for advancing clinical treatments for critical illnesses.
Keywords: critical illness; ferroptosis; iron overload; lipid metabolism; mitochondrial dysfunction.
Copyright © 2024 Tan, Ge, Yan, Guo, Han, Zhu and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mechanisms and preventive measures of ALDH2 in ischemia‑reperfusion injury: Ferroptosis as a novel target (Review).Mol Med Rep. 2025 Apr;31(4):105. doi: 10.3892/mmr.2025.13470. Epub 2025 Feb 28. Mol Med Rep. 2025. PMID: 40017132 Free PMC article. Review.
-
Baicalin alleviates intestinal ischemia-reperfusion injury by regulating ferroptosis mediated by nuclear factor E2-related factor 2/Glutathione peroxidase 4 signaling pathway.J Physiol Pharmacol. 2024 Oct;75(5). doi: 10.26402/jpp.2024.5.07. Epub 2024 Dec 4. J Physiol Pharmacol. 2024. PMID: 39652902
-
Ferroptosis-A New Dawn in the Treatment of Organ Ischemia-Reperfusion Injury.Cells. 2022 Nov 17;11(22):3653. doi: 10.3390/cells11223653. Cells. 2022. PMID: 36429080 Free PMC article. Review.
-
Nuclear factor erythroid 2-related factor-mediated signaling alleviates ferroptosis during cerebral ischemia-reperfusion injury.Biomed Pharmacother. 2024 Nov;180:117513. doi: 10.1016/j.biopha.2024.117513. Epub 2024 Sep 27. Biomed Pharmacother. 2024. PMID: 39341075 Review.
-
Naringenin alleviates intestinal ischemia/reperfusion injury by inhibiting ferroptosis via targeting YAP/STAT3 signaling axis.Phytomedicine. 2024 Dec;135:156095. doi: 10.1016/j.phymed.2024.156095. Epub 2024 Sep 27. Phytomedicine. 2024. PMID: 39383632
Cited by
-
Post-translational modifications orchestrate mTOR-driven cell death in cardiovascular disease.Front Cardiovasc Med. 2025 Jul 15;12:1620669. doi: 10.3389/fcvm.2025.1620669. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40734978 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

