C3 deficiency promotes pulmonary inflammation in AT1R-induced mouse model for systemic sclerosis
- PMID: 39737181
- PMCID: PMC11683138
- DOI: 10.3389/fimmu.2024.1491324
C3 deficiency promotes pulmonary inflammation in AT1R-induced mouse model for systemic sclerosis
Abstract
Introduction: Autoantibody-mediated complement activation plays an essential role in a variety of autoimmune disorders. However, the role of complement in systemic sclerosis (SSc) remains largely unknown. In this study, we aimed to determine the role of complement C3 in the development of a recently described SSc mouse model based on autoimmunity to angiotensin II receptor type 1 (AT1R).
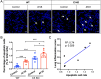
Methods: Mice were immunized with cell membrane extract isolated from Chinese hamster ovary (CHO) cells overexpressing AT1R or non-transfected CHO cells as a control. Peripheral blood, dorsal skin and the lung were then collected to evlauate disease characteristics. Apoptotic cells in the lung of mice were detected using the DeadEnd™ Fluorometric TUNEL System.
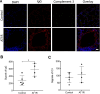
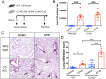
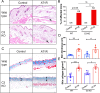
Results: Our results showed that experimental SSc in this model was featured by the deposition of IgG, but not of complement C3, in the lung. After immunization with AT1R, C3-deficient mice developed more severe pulmonary inflammations than wild type controls, whereas skin inflammation and fibrosis were not different as well as the anti-AT1R ab levels. Further, C3-deficient mice showed an increased rate of pulmonary cell apoptosis as compared to controls. The apoptosis rate correlated with the corresponding degree of lung inflammation.
Discussion: Taken together, our findings suggest an anti-apoptotic and anti-inflammatory role of complement C3 in pulmonary autoimmune inflammation.
Keywords: complement; inflammation; mouse model; pulmonary inflammation; systemic sclerosis.
Copyright © 2024 Yin, Verschoor, Yue, Goldmann, Heidecke, Riemekasten, Petersen and Yu.
Conflict of interest statement
HH is the owner and GR is an advisor of the company CellTrend, Germany, which produced the membrane extracts and the tests for the detection of anti-AT1R antibodies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

