Regulation of epidermal barrier function and pathogenesis of psoriasis by serine protease inhibitors
- PMID: 39737188
- PMCID: PMC11683130
- DOI: 10.3389/fimmu.2024.1498067
Regulation of epidermal barrier function and pathogenesis of psoriasis by serine protease inhibitors
Abstract
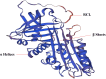
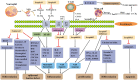
Serine protease inhibitors (Serpins) are a protein superfamily of protease inhibitors that are thought to play a role in the regulation of inflammation, immunity, tumorigenesis, coagulation, blood pressure and cancer metastasis. Serpins is enriched in the skin and play a vital role in modulating the epidermal barrier and maintaining skin homeostasis. Psoriasis is a chronic inflammatory immune-mediated skin disease. At present, most serpins focus on the pathogenesis of psoriasis vulgaris. Only a small number, such as the mutation of SerpinA1/A3/B3, are involved in the pathogenesis of GPP. SerpinA12 and SerpinG1 are significantly elevated in the serum of patients with psoriatic arthritis, but their specific mechanism of action in psoriatic arthritis has not been reported. Some Serpins, including SerpinA12, SerpinB2/B3/B7, play multiple roles in skin barrier function and pathogenesis of psoriasis. The decrease in the expression of SerpinA12, SerpinB7 deficiency and increase in expression of SerpinB3/4 in the skin can promote inflammation and poor differentiation of keratinocyte, with damaged skin barrier. Pso p27, derived from SerpinB3/B4, is an autoantigen that can enhance immune response in psoriasis. SerpinB2 plays a role in maintaining epidermal barrier integrity and inhibiting keratinocyte proliferation. Here we briefly introduce the structure, functional characteristics, expression and distribution of serpins in skin and focus on the regulation of serpins in the epidermal barrier function and the pathogenic role of serpins in psoriasis.
Keywords: hyper-proliferation; inflammation; psoriasis; serine protease inhibitors; skin barrier function.
Copyright © 2024 Wang, Li, Zhou, Hou and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
SERPINB3/B4 Is Increased in Psoriasis and Rosacea Lesions and Has Proinflammatory Effects in Mouse Models of these Diseases.J Invest Dermatol. 2024 Dec;144(12):2706-2718.e6. doi: 10.1016/j.jid.2024.04.011. Epub 2024 May 10. J Invest Dermatol. 2024. PMID: 38735363
-
SerpinB7 deficiency contributes to development of psoriasis via calcium-mediated keratinocyte differentiation dysfunction.Cell Death Dis. 2022 Jul 21;13(7):635. doi: 10.1038/s41419-022-05045-8. Cell Death Dis. 2022. PMID: 35864103 Free PMC article.
-
SERPINB3/B4 contributes to early inflammation and barrier dysfunction in an experimental murine model of atopic dermatitis.J Invest Dermatol. 2015 Jan;135(1):160-169. doi: 10.1038/jid.2014.353. Epub 2014 Aug 11. J Invest Dermatol. 2015. PMID: 25111616 Free PMC article.
-
Psoriasis: the epidermal component.Clin Dermatol. 2007 Nov-Dec;25(6):589-95. doi: 10.1016/j.clindermatol.2007.09.021. Clin Dermatol. 2007. PMID: 18021897 Review.
-
Pso p27, a SERPINB3/B4-derived protein, is most likely a common autoantigen in chronic inflammatory diseases.Clin Immunol. 2017 Jan;174:10-17. doi: 10.1016/j.clim.2016.11.006. Epub 2016 Nov 15. Clin Immunol. 2017. PMID: 27871892 Review.
Cited by
-
Distinct germ-line genetic mutation patterns correlate with reproductive outcomes in ICSI patients: a pilot study.Front Genet. 2025 May 23;16:1610943. doi: 10.3389/fgene.2025.1610943. eCollection 2025. Front Genet. 2025. PMID: 40486681 Free PMC article.
-
Aloe polysaccharide promotes keratinocyte proliferation, migration, and differentiation by upregulating the EGFR/PKC-dependent signaling pathways.Sci Rep. 2025 Mar 10;15(1):8196. doi: 10.1038/s41598-025-91201-x. Sci Rep. 2025. PMID: 40064981 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

