Optimizing immunogenicity and product presentation of a SARS-CoV-2 subunit vaccine composition: effects of delivery route, heterologous regimens with self-amplifying RNA vaccines, and lyophilization
- PMID: 39737197
- PMCID: PMC11683073
- DOI: 10.3389/fimmu.2024.1480976
Optimizing immunogenicity and product presentation of a SARS-CoV-2 subunit vaccine composition: effects of delivery route, heterologous regimens with self-amplifying RNA vaccines, and lyophilization
Abstract
Introduction: Dozens of vaccines have been approved or authorized internationally in response to the ongoing SARS-CoV-2 pandemic, covering a range of modalities and routes of delivery. For example, mucosal delivery of vaccines via the intranasal (i.n.) route has been shown to improve protective mucosal responses in comparison to intramuscular (i.m.) delivery. As we gain knowledge of the limitations of existing vaccines, it is of interest to understand if changes in product presentation or combinations of multiple vaccine modalities can further improve immunological outcomes.
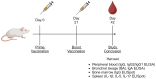
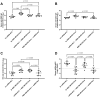
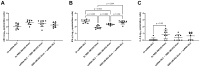
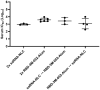
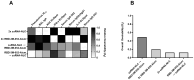
Methods: We investigated a commercial-stage SARS-CoV-2 receptor binding domain (RBD) antigen adjuvanted with a clinical-stage TLR-7/8 agonist (3M-052) formulated on aluminum oxyhydroxide (Alum). In a murine immunogenicity model, we compared i.n. and i.m. dosing of the RBD-3M-052-Alum vaccine. We measured the magnitude of antibody responses in serum and lungs, the antibody-secreting cell populations in bone marrow, and antigen-specific cytokine-secreting splenocyte populations. Similarly, we compared different heterologous and homologous prime-boost regimens using the RBD-3M-052-Alum vaccine and a clinical-stage self-amplifying RNA (saRNA) vaccine formulated on a nanostructured lipid carrier (NLC) using the i.m. route alone. Finally, we developed a lyophilized presentation of the RBD-3M-052-Alum vaccine and compared it to the liquid presentation and a heterologous regimen including a previously characterized lyophilized form of the saRNA-NLC vaccine.
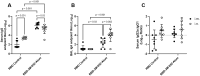
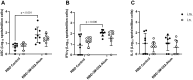
Results and discussion: We demonstrate that i.n. dosing of the RBD-3M-052-Alum vaccine increased IgA titers in the lung by more than 1.5 logs, but induced serum IgG titers 0.8 logs lower, in comparison to i.m. dosing of the same vaccine. We also show that the homologous prime-boost RBD-3M-052-Alum regimen led to the highest serum IgG and bronchial IgA titers, whereas the homologous saRNA-NLC regimen led to the highest splenocyte interferon-γ response. We found that priming with the saRNA-NLC vaccine and boosting with the RBD-3M-052-Alum vaccine led to the most desirable immune outcome of all regimens tested. Finally, we show that the lyophilized RBD-3M-052-Alum vaccine retained its immunological characteristics. Our results demonstrate that the route of delivery and the use of heterologous regimens each separately impacts the resulting immune profile, and confirm that multi-product vaccine regimens can be developed with stabilized presentations in mind.
Keywords: RNA vaccine; adjuvant formulation; heterologous vaccine; intranasal vaccine; lyophilized vaccine; receptor binding domain; vaccine development.
Copyright © 2024 Lykins, Pollet, White, Keegan, Versteeg, Strych, Chen, Mohamath, Ramer-Denisoff, Reed, Renshaw, Beaver, Gerhardt, Voigt, Tomai, Sitrin, Choy, Cassels, Hotez, Bottazzi and Fox.
Conflict of interest statement
The team of scientists at Texas Children’s Hospital Center for Vaccine Development are co-inventors of a COVID-19 recombinant protein vaccine technology owned by Baylor College of Medicine (BCM). The COVID-19 vaccine technology was recently licensed by BCM non-exclusively and with no patent restrictions to several companies committed to advancing vaccines for low- and middle-income countries. The co-inventors have no involvement in license negotiations conducted by BCM. Similar to other research universities, a long-standing BCM policy provides its faculty and staff, who make discoveries that result in a commercial license, a share of any royalty income. Any such distribution is undertaken in accordance with BCM policy. MT is a contract worker for 3M, and 3M-052 is an asset of 3M Health Care. MT is an inventor on patents and/or patent applications involving 3M-052. CF is an inventor on patents and/or patent applications involving formulations of 3M-052 and co-inventor on patents and/or patent applications relating to nanostructured lipid carriers, all of which are assigned to Access to Advanced Health Institute AAHI. AG and EV are co-inventors on patents and/or patent applications relating to thermostable RNA vaccines, all of which are assigned to AAHI. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Afkhami S, D’Agostino MR, Zhang A, Stacey HD, Marzok A, Kang A, et al. . Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. (2022) 185:896–915.e19. doi: 10.1016/j.cell.2022.02.005 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

