Evaluation of the interface of metallic-coated biodegradable polymeric stents with prokaryotic and eukaryotic cells
- PMID: 39737210
- PMCID: PMC11683264
- DOI: 10.1016/j.bioactmat.2024.12.003
Evaluation of the interface of metallic-coated biodegradable polymeric stents with prokaryotic and eukaryotic cells
Abstract
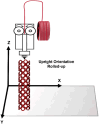
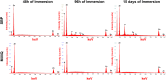
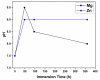
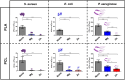
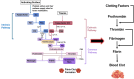
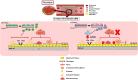
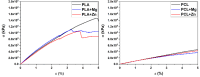
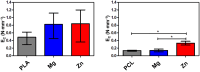
Polymeric coronary stents, like the ABSORB™, are commonly used to treat atherosclerosis due to their bioresorbable and cell-compatible polymer structure. However, they face challenges such as high strut thickness, high elastic recoil, and lack of radiopacity. This study aims to address these limitations by modifying degradable stents produced by additive manufacturing with poly(lactic acid) (PLA) and poly(ε-caprolactone) (PCL) with degradable metallic coatings, specifically zinc (Zn) and magnesium (Mg), deposited via radiofrequency (rf) magnetron sputtering. The characterisation included the evaluation of the degradation of the coatings, antibacterial, anti-thrombogenicity, radiopacity, and mechanical properties. The results showed that the metallic coatings inhibited bacterial growth, though Mg exhibited a high degradation rate. Thrombogenicity studies showed that Zn-coated stents had anticoagulant properties, while Mg-coated and controls were thrombogenic. Zn coatings significantly improved radiopacity, enhancing contrast by 43 %. Mechanical testing revealed that metallic coatings reduced yield strength and, thus, diminished elastic recoil after stent expansion. Zn-coated stents improved cyclic compression resistance by 270 % for PCL stents, with PLA-based stents showing smaller improvements. The coatings also enhanced crush resistance, particularly for Zn-coated PCL stents. Overall, Zn-coated polymers have emerged as the premier prototype due to their superior biological and mechanical performance, appropriate degradation during the stent life, and ability to provide the appropriate radiopacity to medical devices.
Keywords: Biological performance; Mechanical properties; Polymeric bioresorbable stents; Radiopacity; Sputtered metallic coatings.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


























References
-
- Sousa A.M., Amaro A.M., Piedade A.P. Structural design optimization through finite element analysis of additive manufactured bioresorbable polymeric stents. Mater. Today Chem. 2024;36 doi: 10.1016/j.mtchem.2024.101972. - DOI
-
- McMahon S., Bertollo N., Cearbhaill E.D.O., Salber J., Pierucci L., Duffy P., Dürig T., Bi V., Wang W. Bio-resorbable polymer stents: a review of material progress and prospects. Prog. Polym. Sci. 2018;83:79–96. doi: 10.1016/j.progpolymsci.2018.05.002. - DOI
-
- Bink N., Mohan V.B., Fakirov S. Recent advances in plastic stents: a comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2021 doi: 10.1080/00914037.2019.1685519. - DOI
LinkOut - more resources
Full Text Sources

