Comparison of Efficacy of Beractant (SURVANTA®) and Poractant Alfa (CUROSURF®) in Preterm Infants With Respiratory Distress Syndrome in Tawam Hospital, Al Ain, UAE: A Retrospective Study
- PMID: 39737314
- PMCID: PMC11683160
- DOI: 10.7759/cureus.74790
Comparison of Efficacy of Beractant (SURVANTA®) and Poractant Alfa (CUROSURF®) in Preterm Infants With Respiratory Distress Syndrome in Tawam Hospital, Al Ain, UAE: A Retrospective Study
Abstract
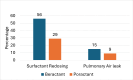
Introduction Respiratory distress syndrome (RDS) is a leading cause of morbidity and mortality among preterm infants, necessitating effective treatment strategies. This study compared the efficacy of Beractant (SURVANTA®) to Poractant alfa (CUROSURF®) in treating RDS in preterm infants admitted to Tawam Hospital in the UAE. Methodology This retrospective study included preterm infants from 23+0 to 36+6 weeks of gestation with a diagnosis of RDS and treatment by Beractant or Poractant alfa within 48 hours of life between January 2020 and March 2023. Data collected from electronic medical records of Tawam Hospital include infant and maternal demographics, primary outcome parameters, such as bronchopulmonary dysplasia (BPD) and/or mortality, and secondary outcome parameters, such as surfactant redosing, air leak syndrome, and other complications. Results A total of 258 infants met the inclusion criteria: 178 were treated with Beractant, and 80 were treated with Poractant alfa. After adjusting the confounding factors, the occurrence of bronchopulmonary dysplasia (BPD) was not statistically significant, showing rates of 68.7% (n=46) in the Poractant group and 47.5% (n=75) in the Beractant group (p=0.71). Likewise, there was no significant difference in mortality rates between the two groups, with 22.5% (n=18) in the Poractant group and 11.8% (n=21) in the Beractant group (p=0.33). Furthermore, the combined incidence of BPD or mortality was also not statistically significant, recorded at 53.4% (n=95) for the Beractant group compared to 73.8% (n=59) for the Poractant group (p=0.93). However, the need for surfactant redosing and air leak syndrome was significantly lower in the Poractant group compared to the Beractant group, 26.2% (n=21) vs 45.5% (n=81), p < 0.00001 and 8.8% (n=7) vs 14.6% (n=26), p = 0.05, respectively. There was no difference in the incidence of other outcomes such as pulmonary hemorrhage, periventricular leukomalacia (PVL), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), significant patent ductus arteriosus (PDA), and retinopathy of prematurity (ROP) that required treatment. Conclusion There was no significant difference in the rates of bronchopulmonary dysplasia or mortality between Poractant alfa (CUROSURF®) and Beractant (SURVANTA®) in preterm infants suffering from respiratory distress syndrome. Poractant alfa (CUROSURF®) showed a reduced need for surfactant redosing and a lower incidence of air leak syndrome. However, the rates of other outcomes, including significant patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), periventricular leukomalacia (PVL), and necrotizing enterocolitis (NEC), were comparable in both treatment groups. Further randomized prospective studies are necessary to evaluate these types of surfactants and investigate their efficacy as well as both short- and long-term outcomes.
Keywords: beractant; bronchopulmonary dysplasia; poractant; preterm infants; respiratory distress syndrome; surfactant therapy.
Copyright © 2024, Butt et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- European consensus guidelines on the management of respiratory distress syndrome—2016 update. Sweet DG, Carnielli V, Greisen G, et al. Neonatology. 2017;111:107–125. - PubMed
-
- Randomized clinical trial comparing two natural surfactant preparations to treat respiratory distress syndrome. Hammoud M, Al-Kazmi N, Alshemmiri M, Thalib L, Ranjani VT, Devarajan LV, Elsori H. J Matern Fetal Neonatal Med. 2004;15:167–175. - PubMed
-
- Surfactant replacement therapy for preterm and term neonates with respiratory distress. Polin RA, Carlo WA. Pediatrics. 2014;133:156–163. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous