Mixed-methods process evaluation of a proactive approach to healthcare in Parkinson's disease-ParkProReakt: a protocol of a hybrid efficacy-implementation study
- PMID: 39737345
- PMCID: PMC11683930
- DOI: 10.1136/bmjno-2024-000966
Mixed-methods process evaluation of a proactive approach to healthcare in Parkinson's disease-ParkProReakt: a protocol of a hybrid efficacy-implementation study
Abstract
Introduction: People with Parkinson's disease (PwPD) experience a wide range of motor and non-motor symptoms that have a significant impact on their health and quality of life. Effective care management for PwPD involves monitoring symptoms at home, involving specialised multidisciplinary care providers and enhancing self-management skills. This study protocol describes the process evaluation within a randomised clinical trial to assess the implementation and its impact on patient health outcomes of ParkProReakt-a proactive, multidisciplinary, digitally supported care model for community-dwelling PwPD.
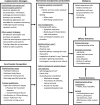
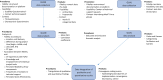
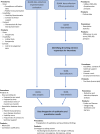
Methods and analysis: The hybrid efficacy-implementation study will assess key implementation outcomes using the Medical Research Council framework for complex interventions alongside a randomised controlled trial. A combination of quantitative and qualitative methods will be used to assess process data from care providers and patients. The main process outcomes are fidelity, dose, feasibility and context. Context will be analysed through semistructured interviews and focus groups using the Consolidated Framework of Implementation Research. To elucidate potential facilitators and barriers to implementation and to gain deeper insights into the efficacy outcome data, quantitative and qualitative process data will be integrated at an interpretative level using mixed methods. In addition to process evaluation, potential indirect mechanisms of impact will be measured.
Ethics and dissemination: Ethical approval for this study was obtained from the responsible state medical ethics committees in Hesse and Hamburg, Germany. Results will be communicated to the funding body and disseminated through scientific publications.
Trial registration: This study was registered with the German Registry for Clinical Studies (DRKS)-number: DRKS00031092.
Keywords: PARKINSON'S DISEASE; QUALITY OF LIFE.
Copyright © Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
NA, MvM, JS, AP, KN, DW, GB, MN, MOA, AP, MI, PMO and US are funded via the ParkProReakt project. IW, DB, RW, KS, NP, FE, ID, KS, VG, BS, MG, XH, HB, MG, LK and CE declare no CoI. EK has received grants from the German Ministry of Education and Research, General Joint Committee, Germany, the German Parkinson Society and STADAPHARM GmbH; honoraria from AbbVie GmbH Germany; memodio GmbH Germany; licence fees from Prolog GmbH, Germany; all outside the submitted work. AKF has received grants from the German Parkinson Society, the German Alzheimer’s Society, the German Parkinson Foundation, STADAPHARM GmbH and the General Joint Committee Germany as well as honoraria from Springer Medizin Verlag GmbH, Heidelberg, Germany; Springer-Verlag GmbH, Berlin; ProLog Wissen GmbH, Cologne, Germany; Seminar- und Fortbildungszentrum Rheine, Germany; LOGOMANIA, Fendt LOGUAN, Ulm, Germany; dbs e.V., Moers, Germany; STADAPHARM GmbH, Bad Vilbel, Germany; NEUROPSY, St. Konrad, Austria; Multiple Sclerosis Society Vienna, Vienna, Austria; and Gossweiler Foundation, Bern, Switzerland. AFK is the author of the cognitive intervention series 'NEUROvitalis' but receives no corresponding honoraria. DP has received honoraria as a speaker at symposia sponsored by Boston Scientific Corp, Medtronic, AbbVie Inc, Zambon and Esteve Pharmaceuticals GmbH. DP received payments as a consultant for Boston Scientific Corp and Bayer and a scientific grant from Boston Scientific Corp. DJP has received honoraria as a speaker at symposia sponsored by Boston Scientific Corp, Medtronic, AbbVie Inc, Zambon and Esteve Pharmaceuticals GmbH. He received payments as a consultant for Boston Scientific Corp and Bayer, and he received a scientific grant from Boston Scientific Corp for a project entitled: 'Sensor-based optimisation of Deep Brain Stimulation settings in Parkinson’s disease' (COMPARE-DBS). Finally, DJP was reimbursed by Esteve Pharmaceuticals GmbH and Boston Scientific Corp for travel expenses to attend congresses. MvM received an honorarium as a speaker at a symposium sponsored by Esteve Pharmaceuticals GmbH. In addition, she was reimbursed travel expenses by Esteve Pharmaceuticals GmbH for attending a congress in 2023.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
