Phase 3 Study of Talazoparib Plus Enzalutamide Versus Placebo Plus Enzalutamide as First-Line Treatment in Patients With Metastatic Castration-Resistant Prostate Cancer: TALAPRO-2 Japanese Subgroup Analysis
- PMID: 39737542
- PMCID: PMC11686335
- DOI: 10.1002/cam4.70333
Phase 3 Study of Talazoparib Plus Enzalutamide Versus Placebo Plus Enzalutamide as First-Line Treatment in Patients With Metastatic Castration-Resistant Prostate Cancer: TALAPRO-2 Japanese Subgroup Analysis
Abstract
Background: In TALAPRO-2, the poly(ADP-ribose) polymerase inhibitor talazoparib plus the androgen receptor-signaling inhibitor enzalutamide improved radiographic progression-free survival (rPFS) versus placebo plus enzalutamide (hazard ratio [HR] = 0.63; 95% CI, 0.51-0.78) in molecularly unselected patients with metastatic castration-resistant prostate cancer (mCRPC). We report an exploratory analysis of efficacy, safety, and pharmacokinetics in Japanese patients enrolled in the TALAPRO-2 study.
Methods: The ongoing, multinational, randomized, double-blind, phase 3 TALAPRO-2 study enrolled patients with mCRPC receiving ongoing androgen deprivation therapy. Patients were prospectively assessed for homologous recombination repair (HRR) gene alterations and randomized 1:1 to receive talazoparib or placebo plus enzalutamide once daily. The primary endpoint was rPFS by blinded independent central review (BICR). Secondary endpoints included overall survival, objective response, safety, and pharmacokinetics.
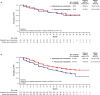
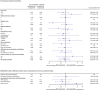
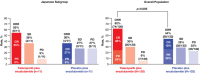
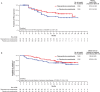
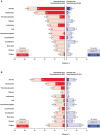
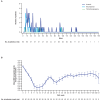
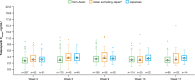
Results: For the 116 Japanese all-comers patients enrolled in TALAPRO-2, the HR for rPFS was 0.89 (95% CI, 0.45-1.75) for the talazoparib versus placebo arm; among those with HRR-deficient disease, the HR was 0.58 (95% CI, 0.16-2.20). Among patients with BRCA1/2 gene alterations in the HRR-deficient population (n = 10), the HR for rPFS was < 0.01 (95% CI, < 0.01-not reached) for the talazoparib versus placebo arm. In the all-comers population, the objective response rate by BICR was 55% (all complete responses) in the talazoparib arm versus 36% in the placebo arm. The safety profile of talazoparib plus enzalutamide was similar between Japanese patients and the overall all-comers population; no new safety signals were identified. Anemia was the most common grade 3/4 treatment-emergent adverse event (55%) and cause of talazoparib discontinuation (12%). Talazoparib Ctrough was comparable across Japanese, Asian, and non-Asian subgroups.
Conclusions: In this exploratory analysis, efficacy outcomes with talazoparib plus enzalutamide in Japanese patients in TALAPRO-2 were consistent with those in the overall all-comers population. The safety profile and pharmacokinetics of the combination were similar between Japanese patients and the overall all-comers population.
Trial registration: ClinicalTrials.gov Identifier: NCT03395197.
Keywords: Japanese; PARP inhibitor; TALAPRO‐2; enzalutamide; mCRPC; talazoparib.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Nobuaki Matsubara reports honoraria (personal) from Sanofi; research funding (institution) from Amgen, Astellas Pharma, AstraZeneca, Bayer, Chugai Pharma, Eisai, Janssen, Lilly, MSD, Pfizer, PRA Health Science, Roche, Seagen, Taiho, and Takeda; and travel, accommodations, and/or expenses (personal) from Pfizer. Hideaki Miyake reports honoraria from Astellas Pharma, AstraZeneca, Bayer, Eisai, Ferring, Janssen, Merck, MSD, Nippon Shinyaku, Ono, Pfizer, Sanofi, and Takeda. Hiroji Uemura reports honoraria from Astellas Pharma, AstraZeneca, Bayer, Ferring, Janssen, Merck, MSD, Ono, Pfizer, Sanofi, Chugai, Novartis, Daiichi Sankyo, Nihon Medi‐Physics, Nippon Shinyaku, and Takeda. Atsushi Mizokami reports honoraria from Janssen, Sanofi, Bayer, Takeda, and PDR Pharma. Hiroaki Kikukawa, Takeo Kosaka, Motonobu Nakamura, Kazuki Kobayashi, and Atsushi Komaru report no conflicts of interest. Kazuo Nishimura reports honoraria from Astellas Pharma, AstraZeneca, Bayer, Janssen, MSD, and Merck. Yuko Mori, Shigeyuki Toyoizumi, Natsuki Hori, and Yoshiko Umeyama are employees of Pfizer R&D Japan and may hold stock in Pfizer, Inc. Hirotsugu Uemura reports receiving research grants from Astellas Pharma, AstraZeneca, Chugai, Janssen, Ono, Osaka Urology Research Foundation, and Takeda; consulting fees from BMS, Ono, and Sanofi; lecture and speaker's fees from Bayer, BMS, Janssen, MSD, Takeda, Sanofi, and Pfizer.
Figures
References
-
- McKay R. R., Morgans A. K., Shore N. D., et al., “First‐Line Combination Treatment With PARP and Androgen Receptor‐Signaling Inhibitors in HRR‐Deficient mCRPC: Applying Clinical Study Findings to Clinical Practice in the United States,” Cancer Treatment Reviews 126 (2024): 102726, 10.1016/j.ctrv.2024.102726. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

