Non-APOE variants predominately expressed in smooth muscle cells contribute to the influence of Alzheimer's disease genetic risk on white matter hyperintensities
- PMID: 39737667
- PMCID: PMC11848156
- DOI: 10.1002/alz.14455
Non-APOE variants predominately expressed in smooth muscle cells contribute to the influence of Alzheimer's disease genetic risk on white matter hyperintensities
Abstract
Introduction: White matter hyperintensity volumes (WMHVs) are disproportionally prevalent in individuals with Alzheimer's disease (AD), potentially reflecting neurovascular injury. We quantify the association between AD polygenic risk score (AD-PRS) and WMHV, exploring single-nucleotide polymorphisms (SNPs) that are proximal to genes overexpressed in cerebrovascular cell species.
Methods: In a UK-Biobank sub-sample (mean age = 64, range = 45-81 years), we associate WMHV with (1) AD-PRS estimated via SNPs across the genome (minus apolipoprotein E [APOE] locus) and (2) AD-PRS estimated with SNPs proximal to specific genes that are overexpressed in cerebrovascular cell species.
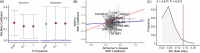
Results: We observed a positive association between non-APOE-AD-PRS and WMHVs. We further demonstrate an association between WMHVs and AD-PRS constructed with SNPs that are proximal to genes over-represented in smooth muscles cells (SMCs; β = 0.135, PFWE < 0.01) and internally replicated (PDISCOVERY+REPLICATION < 0.01).
Discussion: Common AD genetic risk could explain physiological processes underlying vascular pathology in AD. SMC function may offer a treatment target to prevent WMHV-related AD pathophysiology prior to the onset of symptoms.
Highlights: Alzheimer's disease (AD) risk factors such as apolipoprotein E (APOE) ε4, link to increased white matter hyperintensity volume (WMHV). WMHVs indicate vascular risk and neurovascular injury in AD. The broader genetic link between AD risk and WMHV is not fully understood. We quantify AD polygenic risk score (PRS) associations with WMHV, excluding APOE. AD-PRS in smooth muscle cells (SMCs) shows a significant association with increased WMHV.
Keywords: cerebrovascular; polygenic risk score; preclinical; smooth muscles cells; white matter hyperintensities.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
All authors declare that they have no competing interests. Author disclosures are available in the supporting information.
Figures

References
-
- Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer's disease. Nat Neurosci. 2020;23(3):311‐322. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

