The Metapopulation Bridge to Macroevolutionary Speciation Rates: A Conceptual Framework and Empirical Test
- PMID: 39737715
- PMCID: PMC11687345
- DOI: 10.1111/ele.70021
The Metapopulation Bridge to Macroevolutionary Speciation Rates: A Conceptual Framework and Empirical Test
Abstract
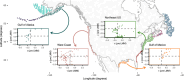
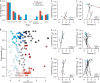
Whether large-scale variation in lineage diversification rates can be predicted by species properties at the population level is a key unresolved question at the interface between micro- and macroevolution. All else being equal, species with biological attributes that confer metapopulation stability should persist more often at timescales relevant to speciation and so give rise to new (incipient) forms that share these biological traits. Here, we develop a framework for testing the relationship between metapopulation properties related to persistence and phylogenetic speciation rates. We illustrate this conceptual approach by applying it to a long-term dataset on demersal fish communities from the North American continental shelf region. We find that one index of metapopulation persistence has phylogenetic signal, suggesting that traits are connected with range-wide demographic patterns. However, there is no relationship between demographic properties and speciation rate. These findings suggest a decoupling between ecological dynamics at decadal timescales and million-year clade dynamics, raising questions about the extent to which population-level processes observable over ecological timescales can be extrapolated to infer biodiversity dynamics more generally.
Keywords: biodiversity; comparative demography; diversification; emergent traits; extinction biology; macroevolution; metacommunity; microevolution; persistence; range size.
© 2024 The Author(s). Ecology Letters published by John Wiley & Sons Ltd.
Figures



Similar articles
-
Phylogenies support out-of-equilibrium models of biodiversity.Ecol Lett. 2015 Apr;18(4):347-56. doi: 10.1111/ele.12415. Epub 2015 Feb 24. Ecol Lett. 2015. PMID: 25711418
-
Barrier Displacement on a Neutral Landscape: Toward a Theory of Continental Biogeography.Syst Biol. 2017 Mar 1;66(2):167-182. doi: 10.1093/sysbio/syw080. Syst Biol. 2017. PMID: 27590192
-
Microevolutionary processes impact macroevolutionary patterns.BMC Evol Biol. 2018 Aug 10;18(1):123. doi: 10.1186/s12862-018-1236-8. BMC Evol Biol. 2018. PMID: 30097006 Free PMC article.
-
Conceptual and empirical bridges between micro- and macroevolution.Nat Ecol Evol. 2023 Aug;7(8):1181-1193. doi: 10.1038/s41559-023-02116-7. Epub 2023 Jul 10. Nat Ecol Evol. 2023. PMID: 37429904 Review.
-
Combining Molecular, Macroevolutionary, and Macroecological Perspectives on the Generation of Diversity.Cold Spring Harb Perspect Biol. 2024 Sep 3;16(9):a041453. doi: 10.1101/cshperspect.a041453. Cold Spring Harb Perspect Biol. 2024. PMID: 38503506 Review.
References
-
- Allmon, W. D. 1992. “A Causal Analysis of Stages in Allopatric Speciation.” In Oxford Surveys in Evolutionary Biology, edited by Futuyma D. and Antonovics J., 219–258. New York, USA: Oxford University Press.
-
- Allmon, W. D. , and Sampson S. D.. 2016. “The Stages of Speciation: A Stepwise Framework for Analysis of Speciation in the Fossil Record.” In Species and Speciation in the Fossil Record, edited by Yacobucci M. M. and Allmon W. D., 121–167. Chicago and London: University of Chicago Press.
-
- Alonso, D. , Pinyol‐Gallemí A., Alcoverro T., and Arthur R.. 2015. “Fish Community Reassembly After a Coral Mass Mortality: Higher Trophic Groups Are Subject to Increased Rates of Extinction.” Ecology Letters 18: 451–461. - PubMed
-
- Anderson, S. A. S. , and Weir J. T.. 2022. “The Role of Divergent Ecological Adaptation During Allopatric Speciation in Vertebrates.” Science 378: 1214–1218. - PubMed

