Sex-dependent molecular landscape of Alzheimer's disease revealed by large-scale single-cell transcriptomics
- PMID: 39737748
- PMCID: PMC11848167
- DOI: 10.1002/alz.14476
Sex-dependent molecular landscape of Alzheimer's disease revealed by large-scale single-cell transcriptomics
Abstract
Introduction: Alzheimer's disease (AD) shows significant sex differences in prevalence and clinical manifestations, but the underlying molecular mechanisms remain unclear.
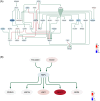
Methods: This study used a large-scale, single-cell transcriptomic atlas of the human prefrontal cortex to investigate sex-dependent molecular changes in AD. Our approach combined cell type-specific and sex-specific differential gene expression analysis, pathway enrichment, gene regulatory network construction, and cell-cell communication analysis to identify sex-dependent changes.
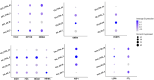
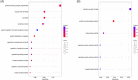
Results: We found significant sex-specific gene expression patterns and pathway alterations in AD. Male astrocytes showed changes in cell death pathways, with RPTOR as a key regulator, while female astrocytes had alterations in Wnt signaling and cell cycle regulation. Cell-cell communication analysis uncovered sex-specific intercellular signaling patterns, with male-specific impacts on apoptosis-related signaling and female-specific alterations in Wnt and calcium signaling.
Discussion: This study reveals sex-dependent gene expression patterns, pathway alterations, and intercellular communication changes, suggesting the need for sex-specific approaches in AD research.
Highlights: Single-cell transcriptomics reveals significant sex-specific molecular signatures in Alzheimer's disease (AD). Male astrocytes show enhanced modulation of apoptotic and cell death pathways in AD; female astrocytes exhibit distinct alterations in Wnt signaling and cell-cycle regulation. Sex-dimorphic changes in mitochondrial gene expression are observed in excitatory neurons, suggesting divergent energy metabolism profiles may contribute to AD sex differences. RPTOR is identified as a key regulator of male-specific changes in astrocytes, implicating the mechanistic target of rapamycin pathway in sex-dependent AD pathology. New cell-cell communication analyses reveal sex-specific patterns of intercellular signaling, providing insights into how cellular interactions may differentially contribute to AD pathology in males and females.
Keywords: Alzheimer's disease; apoptosis; cell death; network analysis; pathway analysis; sex differences; single‐cell sequencing; transcriptome analysis.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors have declared that no competing interests exist. Author disclosures are available in the supporting information.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

