Molecular Epidemiology of Invasive Group B Streptococcus in South Africa, 2019-2020
- PMID: 39737783
- PMCID: PMC11998550
- DOI: 10.1093/infdis/jiae633
Molecular Epidemiology of Invasive Group B Streptococcus in South Africa, 2019-2020
Abstract
Background: Group B Streptococcus (GBS) is a leading cause of neonatal meningitis and sepsis and an important cause of disease in adults. Capsular polysaccharide and protein-based GBS vaccines are currently under development.
Methods: Through national laboratory-based surveillance, invasive GBS isolates were collected from patients of all ages between 2019 and 2020. Phenotypic serotyping and antimicrobial susceptibility testing were conducted, followed by whole-genome sequencing for analysis of population structure and surface protein and resistance genes.
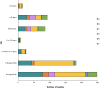
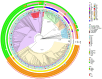
Results: In total, 1748 invasive GBS cases were reported. Of these, 661 isolates underwent characterization, with 658 yielding both phenotypic and genotypic results. Isolates (n = 658) belonged to 5 clonal complexes (CC1, CC8/10, CC17, CC19, and CC23) and 6 serotypes were detected: III (42.8%), Ia (27.9%), V (11.9%), II (8.4%), Ib (6.7%), and IV (2.3%). Phenotypically, only 1 isolate exhibited reduced penicillin susceptibility (minimum inhibitory concentration 0.25 µg/mL). Phenotypic resistance to erythromycin, clindamycin, and tetracycline was observed in 16.1%, 3.8%, and 91.5% of isolates, respectively. ermTR (34.9%) and mefA/E (30.1%) genes were most common among erythromycin-resistant isolates, while ermB predominated in clindamycin-resistant isolates (32.0%). tetM accounted for 95.8% of tetracycline resistance. All isolates carried at least 1 of the 3 pilus gene clusters, 1 of the 4 homologous alpha/Rib family determinants, and 98% harbored 1 of the serine-rich repeat protein genes. hvgA was found exclusively in CC17 isolates.
Conclusions: In our setting, β-lactam antibiotics remain appropriate for GBS treatment and polysaccharide and protein-based vaccines under development are expected to provide good coverage.
Keywords: Streptococcus agalactiae; antibiotic resistance; molecular characterization; population structure; vaccine targets.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. S. M. has participated in an advisory board for GSK on meningococcal B vaccine use in South Africa, unrelated to this article. N. W. has received funding from Bill and Melinda Gates Foundation, World Health Organization, and Sanofi. A. v. G. is the chairperson of the National Advisory Group for Immunisation, South Africa. M. D. has received grant funding from the Bill and Melinda Gates Foundation. S. W. has received grant funding from the Bill and Melinda Gates Foundation, and the Centers for Diseases Control and Prevention. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Lin F-YC, Weisman LE, Troendle J, Adams K. Prematurity is the major risk factor for late-onset group B Streptococcus disease. J Infect Dis 2003; 188:267–71. - PubMed
-
- Verani JR, McGee L, Schrag SJ.. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

