Spatial proteomic differences in chronic traumatic encephalopathy, Alzheimer's disease, and primary age-related tauopathy hippocampi
- PMID: 39737785
- PMCID: PMC11848160
- DOI: 10.1002/alz.14487
Spatial proteomic differences in chronic traumatic encephalopathy, Alzheimer's disease, and primary age-related tauopathy hippocampi
Abstract
Introduction: Alzheimer's disease (AD), primary age-related tauopathy (PART), and chronic traumatic encephalopathy (CTE) all feature hyperphosphorylated tau (p-tau)-immunoreactive neurofibrillary degeneration, but differ in neuroanatomical distribution and progression of neurofibrillary degeneration and amyloid beta (Aβ) deposition.
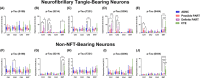
Methods: We used Nanostring GeoMx Digital Spatial Profiling to compare the expression of 70 proteins in neurofibrillary tangle (NFT)-bearing and non-NFT-bearing neurons in hippocampal CA1, CA2, and CA4 subregions and entorhinal cortex of cases with autopsy-confirmed AD (n = 8), PART (n = 7), and CTE (n = 5).
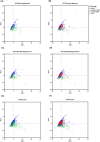
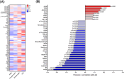
Results: There were numerous subregion-specific differences related to Aβ processing, autophagy/proteostasis, inflammation, gliosis, oxidative stress, neuronal/synaptic integrity, and p-tau epitopes among these different disorders.
Discussion: These results suggest that there are subregion-specific proteomic differences among the neurons of these disorders, which appear to be influenced to a large degree by the presence of hippocampal Aβ. These proteomic differences may play a role in the differing hippocampal p-tau distribution and pathogenesis of these disorders.
Highlights: Alzheimer's disease neuropathologic change (ADNC), possible primary age-related tauopathy (PART), definite PART, and chronic traumatic encephalopathy (CTE) can be differentiated based on the proteomic composition of their neurofibrillary tangle (NFT)- and non-NFT-bearing neurons. The proteome of these NFT- and non-NFT-bearing neurons is largely correlated with the presence or absence of amyloid beta (Aβ). Neurons in CTE and definite PART (Aβ-independent pathologies) share numerous proteomic similarities that distinguish them from ADNC and possible PART (Aβ-positive pathologies).
Keywords: Alzheimer's disease neuropathologic change; aging; autophagy; chronic traumatic encephalopathy; cognitive reserve; neurodegeneration; oxidative stress; primary age‐related tauopathy; resilience; resistance; synapse loss; synapses; tauopathy.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Preliminary results of the data presented in this paper have been published in abstract form for the 2024 Alzheimer's Disease/Parkinson's Disease (AD/PD) conference. The authors declare that they have no competing interests, conflicts of interest, or other relevant disclosures. Author disclosures are available in the supporting information.
Figures



References
-
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63‐75. - PubMed
-
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology. 2007;69:2197‐2204. - PubMed
MeSH terms
Substances
Grants and funding
- R01AG054008/AG/NIA NIH HHS/United States
- R01 AG065839/AG/NIA NIH HHS/United States
- PDF/Parkinson's Disease Foundation/United States
- R01 AG062348/AG/NIA NIH HHS/United States
- RF1AG060961/AG/NIA NIH HHS/United States
- U54 AG079754/AG/NIA NIH HHS/United States
- R01 NS086736/NS/NINDS NIH HHS/United States
- P01 AG060882/AG/NIA NIH HHS/United States
- K01 AG070326/AG/NIA NIH HHS/United States
- R01AG068293/AG/NIA NIH HHS/United States
- RF1 NS095252/NS/NINDS NIH HHS/United States
- R01 AG054008/AG/NIA NIH HHS/United States
- R01NS086736/NS/NINDS NIH HHS/United States
- U54AG079754/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- U54NS115266/NS/NINDS NIH HHS/United States
- 957581/Texas Alzheimer's Research and Care Consortium
- R21 AG078505/AG/NIA NIH HHS/United States
- R01AG062348/AG/NIA NIH HHS/United States
- R21 NS125171/NS/NINDS NIH HHS/United States
- U54 NS115266/NS/NINDS NIH HHS/United States
- RF1NS095252/NS/NINDS NIH HHS/United States
- R01AG065839/AG/NIA NIH HHS/United States
- P30AG066514/AG/NIA NIH HHS/United States
- 957607/Texas Alzheimer's Research and Care Consortium
- R24 AG073199/AG/NIA NIH HHS/United States
- Cure Alzheimer's Fund and Hevolution Foundation/American Federation of Aging Research
- R24AG073199/AG/NIA NIH HHS/United States
- Rainwater Charitable Foundation
- RF1 AG060961/AG/NIA NIH HHS/United States
- K01AG070326/AG/NIA NIH HHS/United States
- R01 AG068293/AG/NIA NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- I01 BX005717/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

