Ferritin-Based Supramolecular Assembly Drug Delivery System for Aminated Fullerene Derivatives to Enhance Tumor-Targeted Therapy
- PMID: 39737845
- PMCID: PMC11848615
- DOI: 10.1002/advs.202413389
Ferritin-Based Supramolecular Assembly Drug Delivery System for Aminated Fullerene Derivatives to Enhance Tumor-Targeted Therapy
Abstract
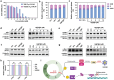
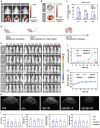
Owing to their attractive antitumor effects, aminated fullerene derivatives are emerging as promising therapeutic drugs for cancer. However, their in vivo applications are severely limited due to cation toxicity. To address this problem, human heavy chain ferritin (HFn), possessing natural biocompatibility is utilized, to develop a novel supramolecular assembly drug delivery system. Specifically, tetra[4-(amino)piperidin-1-yl]-C60 (TAPC) is selected as the representative aminated fullerene, and a layer-by-layer assembly strategy is designed to controllably assemble TAPC with the negatively charged HFn into a hierarchical coassembly (H@T@H) via electrostatic interactions and hydrogen bonds. In this ordered multilayer structure, the surface displayed HFn endows the inner TAPC with biocompatibility, tumor-targeting and blood-brain barrier crossing ability. Additionally, the electrostatic assembly mode enables the acid-responsive disassembly of H@T@H to release TAPC in lysosomes. In the orthotopic glioma mouse model, the HFn-assembled TAPC (H@T@H) shows higher brain accumulation and a stronger inhibitory effect on glioma than polyethylene glycol (PEG)-coated TAPC. Moreover, in an experimental metastasis mouse model, H@T@H have significant preventive and therapeutic effects on tumor metastasis. Encouragingly, the ferritin-based supramolecular assembly strategy has been proven to have broad applicability for various aminated fullerene derivatives, showing promising potential for tackling the in vivo delivery challenges of cationic drugs.
Keywords: aminated fullerene derivative; drug delivery system; ferritin; supramolecular assembly; tumor‐targeted therapy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Tang J., Chen Z., Sun B., Dong J., Liu J., Zhou H., Wang L., Bai R., Miao Q., Zhao Y., Chen C., Liu Y., Nanomedicine. 2016, 12, 945. - PubMed
MeSH terms
Substances
Grants and funding
- 82122037/National Natural Science Foundation of China
- 52272049/National Natural Science Foundation of China
- U23A20686/National Natural Science Foundation of China
- 2021YFA1201102/National Key Research and Development Program of China
- 2022YFA1205900/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
