Downregulation of semaphorin 4A in keratinocytes reflects the features of non-lesional psoriasis
- PMID: 39737847
- PMCID: PMC11687936
- DOI: 10.7554/eLife.97654
Downregulation of semaphorin 4A in keratinocytes reflects the features of non-lesional psoriasis
Abstract
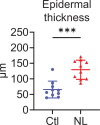
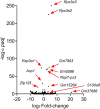
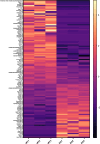
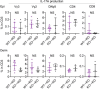
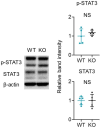
Psoriasis is a multifactorial disorder mediated by IL-17-producing T cells, involving immune cells and skin-constituting cells. Semaphorin 4A (Sema4A), an immune semaphorin, is known to take part in T helper type 1/17 differentiation and activation. However, Sema4A is also crucial for maintaining peripheral tissue homeostasis and its involvement in skin remains unknown. Here, we revealed that while Sema4A expression was pronounced in psoriatic blood lymphocytes and monocytes, it was downregulated in the keratinocytes of both psoriatic lesions and non-lesions compared to controls. Imiquimod application induced more severe dermatitis in Sema4A knockout (KO) mice compared to wild-type (WT) mice. The naïve skin of Sema4A KO mice showed increased T cell infiltration and IL-17A expression along with thicker epidermis and distinct cytokeratin expression compared to WT mice, which are hallmarks of psoriatic non-lesions. Analysis of bone marrow chimeric mice suggested that Sema4A expression in keratinocytes plays a regulatory role in imiquimod-induced dermatitis. The epidermis of psoriatic non-lesion and Sema4A KO mice demonstrated mTOR complex 1 upregulation, and the application of mTOR inhibitors reversed the skewed expression of cytokeratins in Sema4A KO mice. Conclusively, Sema4A-mediated signaling cascades can be triggers for psoriasis and targets in the treatment and prevention of psoriasis.
Keywords: Sema4A; T cells; epidermis; human; immunology; inflammation; keratinocytes; mTOR signaling; mouse; psoriasis; resident memory T cells.
© 2024, Kume et al.
Conflict of interest statement
MK, HK, YM, AT, KY, YN, NO, MT, KT, TK, MW, AK, MF, RW No competing interests declared, SN, SM affiliated with Maruho Co. as an employee, but has declared no conflicts of interest related to this research
Figures















Update of
- doi: 10.1101/2024.04.02.587777
- doi: 10.7554/eLife.97654.1
- doi: 10.7554/eLife.97654.2
References
-
- Arasa J, Terencio MC, Andrés RM, Marín-Castejón A, Valcuende-Cavero F, Payá M, Montesinos MC. Defective induction of COX-2 expression by psoriatic fibroblasts promotes pro-inflammatory activation of macrophages. Frontiers in Immunology. 2019;10:536. doi: 10.3389/fimmu.2019.00536. - DOI - PMC - PubMed
-
- Asrani K, Sood A, Torres A, Georgess D, Phatak P, Kaur H, Dubin A, Talbot CC, Jr, Elhelu L, Ewald AJ, Xiao B, Worley P, Lotan TL. mTORC1 loss impairs epidermal adhesion via TGF-β/Rho kinase activation. The Journal of Clinical Investigation. 2017;127:4001–4017. doi: 10.1172/JCI92893. - DOI - PMC - PubMed
-
- Carvalheiro T, Affandi AJ, Malvar-Fernández B, Dullemond I, Cossu M, Ottria A, Mertens JS, Giovannone B, Bonte-Mineur F, Kok MR, Marut W, Reedquist KA, Radstake TR, García S. Induction of inflammation and fibrosis by semaphorin 4A in systemic sclerosis. Arthritis & Rheumatology. 2019;71:1711–1722. doi: 10.1002/art.40915. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

