The known unknowns of the Hsp90 chaperone
- PMID: 39737863
- PMCID: PMC11687934
- DOI: 10.7554/eLife.102666
The known unknowns of the Hsp90 chaperone
Abstract
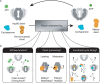
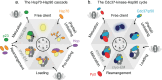
Molecular chaperones are vital proteins that maintain protein homeostasis by assisting in protein folding, activation, degradation, and stress protection. Among them, heat-shock protein 90 (Hsp90) stands out as an essential proteostasis hub in eukaryotes, chaperoning hundreds of 'clients' (substrates). After decades of research, several 'known unknowns' about the molecular function of Hsp90 remain unanswered, hampering rational drug design for the treatment of cancers, neurodegenerative, and other diseases. We highlight three fundamental open questions, reviewing the current state of the field for each, and discuss new opportunities, including single-molecule technologies, to answer the known unknowns of the Hsp90 chaperone.
Keywords: Hsp90; molecular biophysics; molecular chaperones; proteostasis; structural biology.
© 2024, Silbermann, Vermeer et al.
Conflict of interest statement
LS, BV, SS, KT No competing interests declared
Figures


References
-
- Amankwah YS, Fleifil Y, Unruh E, Collins P, Wang Y, Vitou K, Bates A, Obaseki I, Sugoor M, Alao JP, McCarrick RM, Gewirth DT, Sahu ID, Li Z, Lorigan GA, Kravats AN. Structural transitions modulate the chaperone activities of Grp94. PNAS. 2024;121:e2309326121. doi: 10.1073/pnas.2309326121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

