Mitophagy in ischemic heart disease: molecular mechanisms and clinical management
- PMID: 39737905
- PMCID: PMC11685431
- DOI: 10.1038/s41419-024-07303-3
Mitophagy in ischemic heart disease: molecular mechanisms and clinical management
Abstract
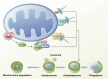
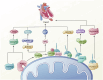
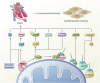
The influence of the mitochondrial control system on ischemic heart disease has become a major focus of current research. Mitophagy, as a very crucial part of the mitochondrial control system, plays a special role in ischemic heart disease, unlike mitochondrial dynamics. The published reviews have not explored in detail the unique function of mitophagy in ischemic heart disease, therefore, the aim of this paper is to summarize how mitophagy regulates the progression of ischemic heart disease. We conclude that mitophagy affects ischemic heart disease by promoting cardiomyocyte hypertrophy and fibrosis, the progression of oxidative stress, the development of inflammation, and cardiomyocyte death, and that the specific mechanisms of mitophagy are worthy of further investigation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
MORN4 protects cardiomyocytes against ischemic injury via MFN2-mediated mitochondrial dynamics and mitophagy.Free Radic Biol Med. 2023 Feb 20;196:156-170. doi: 10.1016/j.freeradbiomed.2023.01.016. Epub 2023 Jan 20. Free Radic Biol Med. 2023. PMID: 36682578
-
Mitochondrial network remodeling of the diabetic heart: implications to ischemia related cardiac dysfunction.Cardiovasc Diabetol. 2024 Jul 18;23(1):261. doi: 10.1186/s12933-024-02357-1. Cardiovasc Diabetol. 2024. PMID: 39026280 Free PMC article. Review.
-
Molecular Signaling to Preserve Mitochondrial Integrity against Ischemic Stress in the Heart: Rescue or Remove Mitochondria in Danger.Cells. 2021 Nov 27;10(12):3330. doi: 10.3390/cells10123330. Cells. 2021. PMID: 34943839 Free PMC article. Review.
-
Ulk1-dependent alternative mitophagy plays a protective role during pressure overload in the heart.Cardiovasc Res. 2022 Sep 20;118(12):2638-2651. doi: 10.1093/cvr/cvac003. Cardiovasc Res. 2022. PMID: 35018428 Free PMC article.
-
MiR-302a-3p aggravates myocardial ischemia-reperfusion injury by suppressing mitophagy via targeting FOXO3.Exp Mol Pathol. 2020 Dec;117:104522. doi: 10.1016/j.yexmp.2020.104522. Epub 2020 Aug 29. Exp Mol Pathol. 2020. PMID: 32866521
Cited by
-
The HNRNPC/CELF2 signaling pathway drives glycolytic reprogramming and mitochondrial dysfunction in drug-resistant acute myeloid leukemia.Cell Biosci. 2025 May 16;15(1):61. doi: 10.1186/s13578-025-01386-x. Cell Biosci. 2025. PMID: 40380235 Free PMC article.
-
Nuclear-localized metabolic enzymes: emerging key players in tumor epigenetic regulation.Mol Cell Biochem. 2025 May 28. doi: 10.1007/s11010-025-05316-w. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40434518 Review.
-
Macrophage Notch1 drives septic cardiac dysfunction by impairing mitophagy and promoting NLRP3 activation.Biol Direct. 2025 May 26;20(1):65. doi: 10.1186/s13062-025-00657-4. Biol Direct. 2025. PMID: 40414862 Free PMC article.
-
TCF7L2 regulates GPX4 to resist ferroptosis and enhance osteogenesis in mouse mesenchymal stem cells.Eur J Med Res. 2025 Aug 5;30(1):713. doi: 10.1186/s40001-025-02993-7. Eur J Med Res. 2025. PMID: 40764595 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

