Anti-CTLA4 treatment reduces lymphedema risk potentially through a systemic expansion of the FOXP3+ Treg population
- PMID: 39737964
- PMCID: PMC11686037
- DOI: 10.1038/s41467-024-55002-6
Anti-CTLA4 treatment reduces lymphedema risk potentially through a systemic expansion of the FOXP3+ Treg population
Abstract
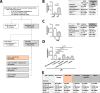
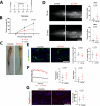
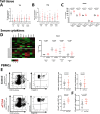
Secondary lymphedema is a common sequel of oncologic surgery and presents a global health burden still lacking pharmacological treatment. The infiltration of the lymphedematous extremities with CD4+T cells influences lymphedema onset and emerges as a promising therapy target. Here, we show that the modulation of CD4+FOXP3+CD25+regulatory T (Treg) cells upon anti-CTLA4 treatment protects against lymphedema development in patients with melanoma and in a mouse lymphedema model. A retrospective evaluation of a melanoma patient registry reveals that anti-CTLA4 reduces lymphedema risk; in parallel, anti-CTLA4 reduces edema and improves lymphatic function in a mouse-tail lymphedema model. This protective effect of anti-CTLA4 correlates with a systemic expansion of Tregs, both in the animal model and in patients with melanoma. Our data thus show that anti-CTLA4 with its lymphedema-protective and anti-tumor properties is a promising candidate for more diverse application in the clinics.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: NL acts as Scientific Advisor and Consultant for Medical Microinstruments (MMI). RD has intermittent, project-focused consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, T3 Pharma, MaxiVAX SA, Pfizer, Simcere and touchIME outside the submitted work. All other authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

