Electrochemically synthesized H2O2 at industrial-level current densities enabled by in situ fabricated few-layer boron nanosheets
- PMID: 39737981
- PMCID: PMC11685507
- DOI: 10.1038/s41467-024-55071-7
Electrochemically synthesized H2O2 at industrial-level current densities enabled by in situ fabricated few-layer boron nanosheets
Abstract
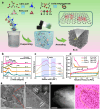
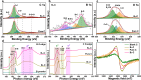
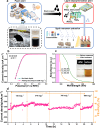
Carbon nanomaterials show outstanding promise as electrocatalysts for hydrogen peroxide (H2O2) synthesis via the two-electron oxygen reduction reaction. However, carbon-based electrocatalysts that are capable of generating H2O2 at industrial-level current densities (>300 mA cm-2) with high selectivity and long-term stability remain to be discovered. Herein, few-layer boron nanosheets are in-situ introduced into a porous carbon matrix, creating a metal-free electrocatalyst (Bn-C) with H2O2 production rates of industrial relevance in neutral or alkaline media. Bn-C maintained > 95% Faradaic efficiency during a 140-hour test at 300 mA cm-2 and 0.1 V vs. RHE, and delivered a mass activity of 25.1 mol gcatalyst-1 h-1 in 1.0 M Na2SO4 using a flow cell. Theoretical simulations and experimental studies demonstrate that the superior catalytic performance originates from B atoms with adsorbed O atoms in the boron nanosheets. Bn-C outperforms all metal-based and metal-free carbon catalysts reported to date for H2O2 synthesis at industrial-level current densities.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests
Figures



References
-
- Li, L. et al. Tailoring selectivity of electrochemical hydrogen peroxide generation by tunable pyrrolic‐nitrogen‐carbon. Adv. Energy Mater.10, 2000789 (2020). - DOI
-
- Chen, S. et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustainable Chem. Eng.6, 311–317 (2018). - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

