Integrated multi-omics profiling reveals neutrophil extracellular traps potentiate Aortic dissection progression
- PMID: 39737994
- PMCID: PMC11686284
- DOI: 10.1038/s41467-024-55038-8
Integrated multi-omics profiling reveals neutrophil extracellular traps potentiate Aortic dissection progression
Abstract
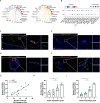
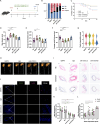
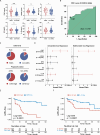
Adverse aortic remodeling increases the risk of aorta-related adverse events (AAEs) after thoracic endovascular aortic repair (TEVAR) and affects the overall prognosis of aortic dissection (AD). It is imperative to delve into the exploration of prognostic indicators to streamline the identification of individuals at elevated risk for postoperative AAEs, and therapeutic targets to optimize the efficacy of TEVAR for patients with AD. Here, we perform proteomic and single-cell transcriptomic analyses of peripheral blood and aortic lesions, respectively, from patients with AD and healthy subjects. The integrated multi-omics profiling identifies that highly phenotype-associated macrophages orchestrate neutrophil extracellular traps (NETs) through CXCL3/CXCR2 axis, thereby promoting the development of AD. Increased NETs formation is a defining feature of systemic immunity and aortic microenvironment of AD. Inhibiting NETs formation through the blockade of citrullinated histone H3 or CXCL3/CXCR2 axis ameliorates the progression and rupture of aortic dissection in male mice. The plasma level of citrullinated histone H3 predicts AAEs following endovascular therapy, facilitating the risk stratification and prognostic evaluation for patients with AD.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Erbel, R. et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J.35, 2873–2926 (2014). - DOI - PubMed
-
- Ogami, T. et al. Long-term outcomes after recurrent acute thoracic aortic dissection: Insights from the International Registry of Aortic Dissection. J. Thorac. Cardiovasc. Surg.24, 283–286 (2024). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

