Alcohol-induced gut microbial reorganization and associated overproduction of phenylacetylglutamine promotes cardiovascular disease
- PMID: 39738016
- PMCID: PMC11685538
- DOI: 10.1038/s41467-024-55084-2
Alcohol-induced gut microbial reorganization and associated overproduction of phenylacetylglutamine promotes cardiovascular disease
Abstract
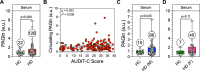
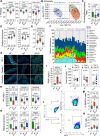
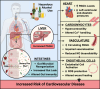
The mechanism(s) underlying gut microbial metabolite (GMM) contribution towards alcohol-mediated cardiovascular disease (CVD) is unknown. Herein we observe elevation in circulating phenylacetylglutamine (PAGln), a known CVD-associated GMM, in individuals living with alcohol use disorder. In a male murine binge-on-chronic alcohol model, we confirm gut microbial reorganization, elevation in PAGln levels, and the presence of cardiovascular pathophysiology. Fecal microbiota transplantation from pair-/alcohol-fed mice into naïve male mice demonstrates the transmissibility of PAGln production and the CVD phenotype. Independent of alcohol exposure, pharmacological-mediated increases in PAGln elicits direct cardiac and vascular dysfunction. PAGln induced hypercontractility and altered calcium cycling in isolated cardiomyocytes providing evidence of improper relaxation which corresponds to elevated filling pressures observed in vivo. Furthermore, PAGln directly induces vascular endothelial cell activation through induction of oxidative stress leading to endothelial cell dysfunction. We thus reveal that the alcohol-induced microbial reorganization and resultant GMM elevation, specifically PAGln, directly contributes to CVD.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Z.W. reports being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Z.W. also reports having received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics and Procter & Gamble. C.M.T. consults for Abbott Laboratories. S.L.H. reports being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, being a paid consultant for Procter & Gamble and Zehna Therapeutics, having received research funds from Procter & Gamble, Zehna Therapeutics, and Roche Diagnostics, and being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab and Procter & Gamble. All other authors: Z.L., M.G., A.Z., X.F., K.M., M.M., X.S.L., Z.Y., J.M., T.T., K.C., M.H., L.H., K.G., T.T.V., D.W., A.M.G., P.Q., C.A.M., J.M.B., D.J.L., D.A.W., and T.E.S. have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HL160569/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- T32 HL069766/HL/NHLBI NIH HHS/United States
- RF1 NS133812/NS/NINDS NIH HHS/United States
- U01 AA026917/AA/NIAAA NIH HHS/United States
- R01 AA029984/AA/NIAAA NIH HHS/United States
- P01 HL147823/HL/NHLBI NIH HHS/United States
- T32 AR065972/AR/NIAMS NIH HHS/United States
- HL171221/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P50 AA024333/AA/NIAAA NIH HHS/United States
- NSF 2018936/National Science Foundation (NSF)
- R01 HL146098/HL/NHLBI NIH HHS/United States
- UH2 AA026226/AA/NIAAA NIH HHS/United States
- R01 DK120679/DK/NIDDK NIH HHS/United States
- KC2210163/U.S. Department of Defense (United States Department of Defense)
- R21 AA027199/AA/NIAAA NIH HHS/United States
- R01AA030312/U.S. Department of Health & Human Services | NIH | National Institute on Alcohol Abuse and Alcoholism (NIAAA)
- P20 GM103424/GM/NIGMS NIH HHS/United States
- R01 DK130227/DK/NIDDK NIH HHS/United States
- P60 AA009803/AA/NIAAA NIH HHS/United States
- R01 HL103866/HL/NHLBI NIH HHS/United States
- R25 HL145817/HL/NHLBI NIH HHS/United States
- UH3 AA026903/AA/NIAAA NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- R01 HL167831/HL/NHLBI NIH HHS/United States
- U24 DK1132746/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 HL151398/HL/NHLBI NIH HHS/United States
- R01 HL146514/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

