Artificial intelligence-driven rational design of ionizable lipids for mRNA delivery
- PMID: 39738043
- PMCID: PMC11685617
- DOI: 10.1038/s41467-024-55072-6
Artificial intelligence-driven rational design of ionizable lipids for mRNA delivery
Abstract
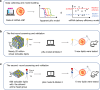
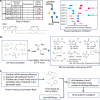
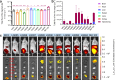
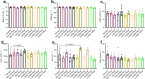
Lipid nanoparticles (LNPs) have proven effective in mRNA delivery, as evidenced by COVID-19 vaccines. Its key ingredient, ionizable lipids, is traditionally optimized by inefficient and costly experimental screening. This study leverages artificial intelligence (AI) and virtual screening to facilitate the rational design of ionizable lipids by predicting two key properties of LNPs, apparent pKa and mRNA delivery efficiency. Nearly 20 million ionizable lipids were evaluated through two iterations of AI-driven generation and screening, yielding three and six new molecules, respectively. In mouse test validation, one lipid from the initial iteration, featuring a benzene ring, demonstrated performance comparable to the control DLin-MC3-DMA (MC3). Notably, all six lipids from the second iteration equaled or outperformed MC3, with one exhibiting efficacy akin to a superior control lipid SM-102. Furthermore, the AI model is interpretable in structure-activity relationships.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- World Health Organization. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... (2022).
-
- Semple, S. C. et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta BBA - Biomembr.1510, 152–166 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SGDX20210823103802016/Shenzhen Science and Technology Innovation Commission
- MYRG-CRG2022-00008-ICMS/Universidade de Macau (University of Macau)
- ICMS/RTO/EP160/2023/Universidade de Macau (University of Macau)
- 0071/2024/RIA1/Fundo para o Desenvolvimento das Ciências e da Tecnologia (Science and Technology Development Fund)
- 005/2023/SKL/Fundo para o Desenvolvimento das Ciências e da Tecnologia (Science and Technology Development Fund)
LinkOut - more resources
Full Text Sources

