Minimal presynaptic protein machinery governing diverse kinetics of calcium-evoked neurotransmitter release
- PMID: 39738049
- PMCID: PMC11685451
- DOI: 10.1038/s41467-024-54960-1
Minimal presynaptic protein machinery governing diverse kinetics of calcium-evoked neurotransmitter release
Abstract
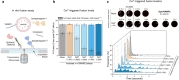
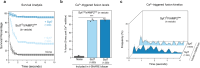
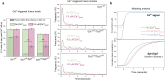
Neurotransmitters are released from synaptic vesicles with remarkable precision in response to presynaptic calcium influx but exhibit significant heterogeneity in exocytosis timing and efficacy based on the recent history of activity. This heterogeneity is critical for information transfer in the brain, yet its molecular basis remains poorly understood. Here, we employ a biochemically-defined fusion assay under physiologically relevant conditions to delineate the minimal protein machinery sufficient to account for various modes of calcium-triggered vesicle fusion dynamics. We find that Synaptotagmin-1, Synaptotagmin-7, and Complexin synergistically restrain SNARE complex assembly, thus preserving vesicles in a stably docked state at rest. Upon calcium activation, Synaptotagmin-1 induces rapid vesicle fusion, while Synaptotagmin-7 mediates delayed fusion. Competitive binding of Synaptotagmin-1 and Synaptotagmin-7 to the same SNAREs, coupled with differential rates of calcium-triggered fusion clamp reversal, govern the overall kinetics of vesicular fusion. Under conditions mimicking sustained neuronal activity, the Synaptotagmin-7 fusion clamp is destabilized by the elevated basal calcium concentration, thereby enhancing the synchronous component of fusion. These findings provide a direct demonstration that a small set of proteins is sufficient to account for how nerve terminals adapt and regulate the calcium-evoked neurotransmitter exocytosis process to support their specialized functions in the nervous system.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

