Nesfatin-1 as a crucial mediator of glucose homeostasis in the reptile, Hemidactylus flaviviridis
- PMID: 39738077
- PMCID: PMC11686144
- DOI: 10.1038/s41598-024-74371-y
Nesfatin-1 as a crucial mediator of glucose homeostasis in the reptile, Hemidactylus flaviviridis
Abstract
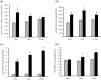
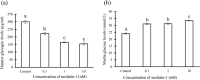
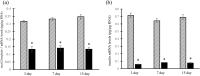
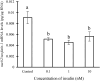
Nesfatin-1 is a crucial regulator of energy homeostasis in mammals and fishes, however, its metabolic role remains completely unexplored in amphibians, reptiles, and birds. Therefore, present study elucidates role of nesfatin-1 in glucose homeostasis in wall lizard wherein fasting stimulated hepatic nucb2/nesfatin-1, glycogen phosphorylase (glyp), phosphoenolpyruvate carboxykinase (pepck), and fructose 1,6-bisphosphatase (fbp), while feeding upregulated pancreatic nucb2/nesfatin-1 and insulin, suggesting towards tissue-specific dual role of nesfatin-1 in glucoregulation. The glycogenolytic/gluconeogenic role of nesfatin-1 was further confirmed by an increase in media glucose levels along with heightened hepatic pepck and fbp expression and concomitant decline in liver glycogen content in nesfatin-1-treated liver of wall lizard. Moreover, treatment with nesfatin-1 stimulated insulin expression in pancreas while insulin downregulated pancreatic nucb2/nesfatin-1. Further, prolonged fasting induced elevated nucb2/nesfatin-1, and lipolytic markers, adipose triglyceride lipase (atgl) and monoglyceride lipase (mgl) in adipose tissue implicate nesfatin-1 in lipolysis which is substantiated by nesfatin-1-mediated direct upregulation of atgl and mgl. Our study provides the first comprehensive overview of tissue-dependent role of nesfatin-1 in regulating energy homeostasis in a reptile.
Keywords: Carbohydrate metabolism; Glucoregulation; Lipolysis; Nesfatin-1; Wall lizard.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Nesfatin-1 in a reptile: its role and hormonal regulation in wall lizard testis.Gen Comp Endocrinol. 2023 Sep 15;341:114337. doi: 10.1016/j.ygcen.2023.114337. Epub 2023 Jun 20. Gen Comp Endocrinol. 2023. PMID: 37348681
-
Ovarian nesfatin-1 in Hemidactylus flaviviridis: Reproductive phase-dependent expression, role and hormonal regulation.Comp Biochem Physiol A Mol Integr Physiol. 2024 Feb;288:111556. doi: 10.1016/j.cbpa.2023.111556. Epub 2023 Nov 26. Comp Biochem Physiol A Mol Integr Physiol. 2024. PMID: 38016591
-
Intestinal NUCB2/nesfatin-1 regulates hepatic glucose production via the MC4R-cAMP-GLP-1 pathway.EMBO J. 2025 Jan;44(1):54-74. doi: 10.1038/s44318-024-00300-4. Epub 2024 Nov 19. EMBO J. 2025. PMID: 39562740 Free PMC article.
-
Role of NUCB2/nesfatin-1 in glucose control: diverse functions in islets, adipocytes and brain.Curr Pharm Des. 2013;19(39):6960-5. doi: 10.2174/138161281939131127130112. Curr Pharm Des. 2013. PMID: 23537085 Review.
-
Nesfatin-1: functions and physiology of a novel regulatory peptide.J Endocrinol. 2017 Jan;232(1):R45-R65. doi: 10.1530/JOE-16-0361. Epub 2016 Oct 17. J Endocrinol. 2017. PMID: 27754932 Review.
References
-
- Oh-I, S. et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443(7112), 709–712 (2006). - PubMed
-
- Feijoo-Bandin, S. et al. Nesfatin-1: A new energy-regulating peptide with pleiotropic functions Implications at cardiovascular level. Endocrine 52, 11–29 (2016). - PubMed
-
- Dotania, K., Tripathy, M. & Rai, U. A comparative account of nesfatin-1 in vertebrates. Gen. Comp. Endocrinol. 312, 113874 (2021). - PubMed
-
- Shimizu, H., Ohsaki, A., Oh, S., Okada, S. & Mori, M. A new anorexigenic protein, nesfatin-1. Peptides 30(5), 995–998 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

