Epidemic Zika virus strains from the Asian lineage induce an attenuated fetal brain pathogenicity
- PMID: 39738084
- PMCID: PMC11686291
- DOI: 10.1038/s41467-024-55155-4
Epidemic Zika virus strains from the Asian lineage induce an attenuated fetal brain pathogenicity
Abstract
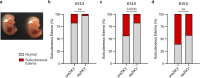
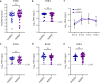
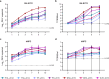
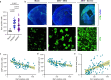
The 2015-2016 Zika virus (ZIKV) outbreak in the Americas revealed the ability of ZIKV from the Asian lineage to cause birth defects, generically called congenital Zika syndrome (CZS). Notwithstanding the long circulation history of Asian ZIKV, no ZIKV-associated CZS cases were reported prior to the outbreaks in French Polynesia (2013) and Brazil (2015). Whether the sudden emergence of CZS resulted from an evolutionary event of Asian ZIKV has remained unclear. We performed a comparative analysis of the pathogenicity of pre-epidemic and epidemic Asian ZIKV strains in mouse embryonic brains using a female immunocompetent intraplacental infection mouse model. All studied Asian ZIKV strains are neurovirulent, but pre-epidemic strains are consistently more pathogenic in the embryos than their epidemic equivalents. Pathogenicity is not directly linked to viral replication. By contrast, an influx of macrophages/microglial cells is noted in infected fetal brains for both pre-epidemic and epidemic ZIKV strains. Moreover, all tested ZIKV strains trigger an immunological response, whereby the intensity of the response differs between strains, and with epidemic ZIKV strains generally mounting a more attenuated immunostimulatory response. Our study reveals that Asian ZIKV strains evolved towards pathogenic attenuation, potentially resulting in CZS emergence in neonates rather than premature death in utero.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Fetal Brain Infection Is Not a Unique Characteristic of Brazilian Zika Viruses.Viruses. 2018 Oct 3;10(10):541. doi: 10.3390/v10100541. Viruses. 2018. PMID: 30282919 Free PMC article.
-
Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains.Nat Commun. 2021 Feb 10;12(1):916. doi: 10.1038/s41467-021-21199-z. Nat Commun. 2021. PMID: 33568638 Free PMC article.
-
Zika viruses of African and Asian lineages cause fetal harm in a mouse model of vertical transmission.PLoS Negl Trop Dis. 2019 Apr 17;13(4):e0007343. doi: 10.1371/journal.pntd.0007343. eCollection 2019 Apr. PLoS Negl Trop Dis. 2019. PMID: 30995223 Free PMC article.
-
Animal models of congenital zika syndrome provide mechanistic insight into viral pathogenesis during pregnancy.PLoS Negl Trop Dis. 2020 Oct 22;14(10):e0008707. doi: 10.1371/journal.pntd.0008707. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33091001 Free PMC article. Review.
-
Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic.Braz J Med Biol Res. 2016;49(5):e5420. doi: 10.1590/1414-431X20165420. Epub 2016 Apr 29. Braz J Med Biol Res. 2016. PMID: 27143174 Free PMC article. Review.
Cited by
-
Neonates at Risk: Understanding the Impact of High-Risk Pregnancies on Neonatal Health.Medicina (Kaunas). 2025 Jun 11;61(6):1077. doi: 10.3390/medicina61061077. Medicina (Kaunas). 2025. PMID: 40572764 Free PMC article. Review.
-
The human neural cell atlas of Zika virus infection in developing brain tissue.Cell Rep Med. 2025 Jun 17;6(6):102189. doi: 10.1016/j.xcrm.2025.102189. Cell Rep Med. 2025. PMID: 40532660 Free PMC article.
References
-
- Wikan, N. & Smith, D. R. First published report of Zika virus infection in people: Simpson, not MacNamara. Lancet Infect. Dis.17, 15–17 (2017). - PubMed
-
- World Health Organization. Zika virus. Factsheet. WHO, Geneva, Switzerland. (2022). https://www.who.int/news-room/fact-sheets/detail/zika-virus
Publication types
MeSH terms
Grants and funding
- 734584/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 734548/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 734584/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 734548/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources
Medical

