Aza-[4 + 2]-cycloaddition of benzocyclobutenones into isoquinolinone derivatives enabled by photoinduced regio-specific C-C bond cleavage
- PMID: 39738103
- PMCID: PMC11685906
- DOI: 10.1038/s41467-024-55110-3
Aza-[4 + 2]-cycloaddition of benzocyclobutenones into isoquinolinone derivatives enabled by photoinduced regio-specific C-C bond cleavage
Abstract
The activation of C-C bond of benzocyclobutenones under mild reaction conditions remains a challenge. We herein report a photoinduced catalyst-free regio-specific C1-C8 bond cleavage of benzocyclobutenones, enabling the generation of versatile ortho-quinoid ketene methides for aza-[4 + 2]-cycloaddition with imines, which offers a facile route to isoquinolinone derivatives, including seven family members of protoberberine alkaloids, gusanlung A, B, D, 8-oxotetrahydroplamatine, tetrahydrothalifendine, tetrahydropalmatine, and xylopinine. Furthermore, the catalytic enantioselective version of this strategy is also realized by merging synergistic photocatalysis and chiral Lewis acid catalysis. Mechanistic studies provide compelling evidence to rationalize the photoisomerization/cycloaddition cascade process.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

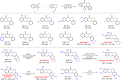

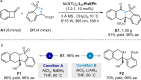
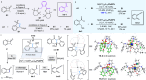
References
-
- Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev.114, 8613–8661 (2014). - PubMed
-
- Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev.115, 9410–9464 (2015). - PubMed
-
- Murakami, M. & Ishida, N. Potential of metal-catalyzed C–C single bond cleavage for organic synthesis. J. Am. Chem. Soc.138, 13759–13769 (2016). - PubMed
-
- Fumagalli, G., Stanton, S. & Bower, J. F. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev.117, 9404–9432 (2017). - PubMed
-
- Kim, D.-S., Park, W.-J. & Jun, C.-H. Metal–organic cooperative catalysis in C–H and C–C bond activation. Chem. Rev.117, 8977–9015 (2017). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

