Age-dependent regulation of axoglial interactions and behavior by oligodendrocyte AnkyrinG
- PMID: 39738113
- PMCID: PMC11686269
- DOI: 10.1038/s41467-024-55209-7
Age-dependent regulation of axoglial interactions and behavior by oligodendrocyte AnkyrinG
Abstract
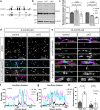
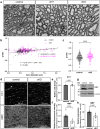
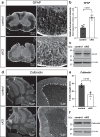
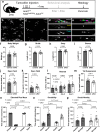
The bipolar disorder (BD) risk gene ANK3 encodes the scaffolding protein AnkyrinG (AnkG). In neurons, AnkG regulates polarity and ion channel clustering at axon initial segments and nodes of Ranvier. Disruption of neuronal AnkG causes BD-like phenotypes in mice. During development, AnkG is also expressed at comparable levels in oligodendrocytes and facilitates the efficient assembly of paranodal junctions. However, the physiological roles of glial AnkG in the mature nervous system, and its contributions to BD-like phenotypes, remain unexplored. Here, we show that oligodendroglia-specific AnkG conditional knockout results in destabilization of axoglial interactions in aged but not young adult mice. In addition, these mice exhibit significant histological, electrophysiological, and behavioral pathophysiologies. Unbiased translatomic profiling reveals potential compensatory machineries. These results highlight the functions of glial AnkG in maintaining proper axoglial interactions throughout aging and suggest a contribution of glial AnkG to neuropsychiatric disorders.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Update of
-
Age-dependent regulation of axoglial interactions and behavior by oligodendrocyte AnkyrinG.bioRxiv [Preprint]. 2024 Apr 2:2024.04.01.587609. doi: 10.1101/2024.04.01.587609. bioRxiv. 2024. Update in: Nat Commun. 2024 Dec 30;15(1):10865. doi: 10.1038/s41467-024-55209-7. PMID: 38617359 Free PMC article. Updated. Preprint.
References
-
- Rasband, M. N. & Peles, E. Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci.22, 7–20 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

