The chromatin remodeling factor OsINO80 promotes H3K27me3 and H3K9me2 deposition and maintains TE silencing in rice
- PMID: 39738209
- PMCID: PMC11686384
- DOI: 10.1038/s41467-024-55387-4
The chromatin remodeling factor OsINO80 promotes H3K27me3 and H3K9me2 deposition and maintains TE silencing in rice
Abstract
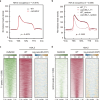
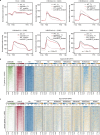
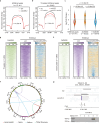
The INO80 chromatin remodeling complex plays a critical role in shaping the dynamic chromatin environment. The diverse functions of the evolutionarily conserved INO80 complex have been widely reported. However, the role of INO80 in modulating the histone variant H2A.Z is controversial. Moreover, whether INO80 helps regulate heterochromatin remains unknown. Here, we characterize the regulatory effects of OsINO80 on protein-coding genes and transposable elements (TEs) in rice. Upon OsINO80 overexpression in rice, we found three types of OsINO80-occupied regions with different chromatin signatures: type I (enriched with H2A.Z), type II (enriched with H3K9me2), and type III (deficient in H2A.Z/H3K9me2). Loss of OsINO80 results in a decrease in H3K27me3, but not H2A.Z, at type I regions as well as a decrease in H3K9me2 at type II regions, which correlates with TE activation and transposition. Our findings reveal that OsINO80 facilitates H3K27me3 establishment, promotes H3K9me2 deposition, and maintains TE silencing.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Chromatin-remodeling factor OsINO80 is involved in regulation of gibberellin biosynthesis and is crucial for rice plant growth and development.J Integr Plant Biol. 2018 Feb;60(2):144-159. doi: 10.1111/jipb.12603. Epub 2018 Jan 5. J Integr Plant Biol. 2018. PMID: 29045007
-
The chromatin remodeler DDM1 prevents transposon mobility through deposition of histone variant H2A.W.Nat Cell Biol. 2021 Apr;23(4):391-400. doi: 10.1038/s41556-021-00658-1. Epub 2021 Apr 8. Nat Cell Biol. 2021. PMID: 33833428
-
OsChz1 acts as a histone chaperone in modulating chromatin organization and genome function in rice.Nat Commun. 2020 Nov 11;11(1):5717. doi: 10.1038/s41467-020-19586-z. Nat Commun. 2020. PMID: 33177521 Free PMC article.
-
DDM1 Maintains Heterochromatin by Regulating Histone Variants.Int J Mol Sci. 2025 May 19;26(10):4845. doi: 10.3390/ijms26104845. Int J Mol Sci. 2025. PMID: 40429984 Free PMC article. Review.
-
Contribution of the histone variant H2A.Z to expression of responsive genes in plants.Semin Cell Dev Biol. 2023 Feb 15;135:85-92. doi: 10.1016/j.semcdb.2022.04.006. Epub 2022 Apr 23. Semin Cell Dev Biol. 2023. PMID: 35474148 Free PMC article. Review.
Cited by
-
Advances in spatial transcriptomics and its application in the musculoskeletal system.Bone Res. 2025 May 16;13(1):54. doi: 10.1038/s41413-025-00429-w. Bone Res. 2025. PMID: 40379648 Free PMC article. Review.
-
The linguistic feedback of tourism robots significantly influences visitors' ecotourism behaviors.Sci Rep. 2025 May 8;15(1):16015. doi: 10.1038/s41598-025-01045-8. Sci Rep. 2025. PMID: 40341673 Free PMC article.
-
The multitalented TIP60 chromatin remodeling complex: wearing many hats in epigenetic regulation, cell division and diseases.Epigenetics Chromatin. 2025 Jul 2;18(1):40. doi: 10.1186/s13072-025-00603-8. Epigenetics Chromatin. 2025. PMID: 40604793 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- SRA/PRJNA1093549
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

