Modeling predicts facile release of nitrite but not nitric oxide from the thionitrate CH3SNO2 with relevance to nitroglycerin bioactivation
- PMID: 39738238
- PMCID: PMC11685828
- DOI: 10.1038/s41598-024-80230-7
Modeling predicts facile release of nitrite but not nitric oxide from the thionitrate CH3SNO2 with relevance to nitroglycerin bioactivation
Abstract
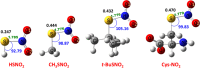
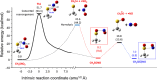
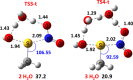
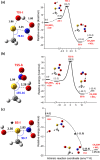
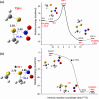
Nitroglycerin is a potent vasodilator in clinical use since the late 1800s. It functions as a prodrug that is bioactivated by formation of an enzyme-based thionitrate, E-Cys-NO2. This intermediate reportedly decomposes to release NO and NO2- but their relative yields remain controversial. Hence, we determined barriers for NO and NO2- production from the model thionitrate, CH3SNO2, using comprehensive high-level quantum chemistry calculations [CCSD(T)//MP2/aug-cc-pVTZ]. We find that the sulfenyl nitrite, CH3SONO, readily releases NO on (S)O-N bond homolysis but CH3SONO formation from CH3SNO2 either by S-NO2 bond homolysis or concerted rearrangement faces prohibitively high barriers (ΔHcalc/ΔH‡calc > 42 kcal/mol). Dramatically lower barriers (ΔH‡calc ~ 17-21 kcal/mol) control NO2- release from CH3SNO2 by gas-phase hydrolysis or nucleophilic attack by OH- or CH3S- on the sulfur atom within the C-S-NO2 molecular plane. Moreover, attack by either anion along the S-NO2 bond results in barrierless NO2- release (ΔH‡calc ~ 0 kcal/mol) since a σ-hole (i.e., area of positive electrostatic potential) extends from this bond. Consistent with our high-level calculations, ALDH2 and GAPDH, enzymes implicated in nitroglycerin bioactivation via an E-Cys-NO2 intermediate, catalyze mainly or exclusively NO2- release from the prodrug.
Keywords: Bioactivation; Nitric oxide; Nitrite; Nitroglycerin; Thionitrate; Vasodilation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Yeates, R. A., Laufen, H. & Leitold, M. The reaction between organic nitrates and sulfhydryl compounds. A possible model system for the activation of organic nitrates. Mol. Pharmacol.28(6), 555–559 (1985). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

