SOCS domain targets ECM assembly in lung fibroblasts and experimental lung fibrosis
- PMID: 39738247
- PMCID: PMC11686354
- DOI: 10.1038/s41598-024-83187-9
SOCS domain targets ECM assembly in lung fibroblasts and experimental lung fibrosis
Abstract
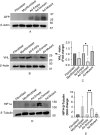
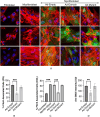
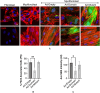
Idiopathic pulmonary fibrosis (IPF) is a fatal disease defined by a progressive decline in lung function due to scarring and accumulation of extracellular matrix (ECM) proteins. The SOCS (Suppressor Of Cytokine Signaling) domain is a 40 amino acid conserved domain known to form a functional ubiquitin ligase complex targeting the Von Hippel Lindau (VHL) protein for proteasomal degradation. Here we show that the SOCS conserved domain operates as a molecular tool, to disrupt collagen and fibronectin fibrils in the ECM associated with fibrotic lung myofibroblasts. Our results demonstrate that fibroblasts differentiated using TGFβ, followed by transduction with the SOCS domain, exhibit significantly reduced levels of the contractile myofibroblast-marker, α-SMA. Furthermore, in support of its role to retard differentiation, we find that lung fibroblasts expressing the SOCS domain present with significantly reduced levels of α-SMA and fibrillar fibronectin after differentiation with TGFβ. We show that adenoviral delivery of the SOCS domain in the fibrotic phase of experimental lung fibrosis in mice, significantly reduces collagen accumulation in disease lungs. These data underscore a novel function for the SOCS domain and its potential in ameliorating pathologic matrix deposition in lung fibroblasts and experimental lung fibrosis.
Keywords: ECM; Fibroblast; Fibronectin; Myofibroblast; SOCS domain.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
SOCS domain targets ECM assembly in lung fibroblasts and experimental lung fibrosis.bioRxiv [Preprint]. 2024 Feb 15:2024.02.14.580347. doi: 10.1101/2024.02.14.580347. bioRxiv. 2024. Update in: Sci Rep. 2024 Dec 30;14(1):31855. doi: 10.1038/s41598-024-83187-9. PMID: 38469152 Free PMC article. Updated. Preprint.
References
-
- Perez, A., Rogers, R. M. & Dauber, J. H. The prognosis of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol.29, S19-26 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

