Impact of Acacia-derived biochar to mitigate salinity stress in Zea mays L. by morpho-physiological and biochemical indices
- PMID: 39738274
- PMCID: PMC11685397
- DOI: 10.1038/s41598-024-83010-5
Impact of Acacia-derived biochar to mitigate salinity stress in Zea mays L. by morpho-physiological and biochemical indices
Abstract
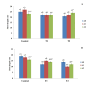
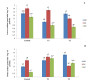
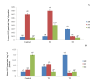
Climate change has caused many challenges to soil ecosystems, including soil salinity. Consequently, many strategies are advised to mitigate this issue. In this context, biochar is acknowledged as a useful addition that can alleviate the detrimental impacts of salt stress on plants. The objective of this study is to evaluate the effects of different levels of salt (Control; T0 0 gl-1, T1; 1.50, and T2; 3 gl-1) and biochar addition rates (A0; 0 g kg-1, A1; 40 g kg-1, and A2; 80 g kg-1) on the agronomic, physiological, and biochemical responses of corn plants. The results of our study showed a significant increase in the biomass of corn plants when exposed to salt stress and treated with 40 g kg-1 of biochar. The result underscores the significant function of Acacia-biochar in mitigating salt toxicity. The application of A1 biochar at a specified rate mitigated the adverse effects of salt-induced oxidative stress by augmenting the activities of catalase (CAT) and glutathione-S-transferase (GST). Furthermore, the utilization of biochar led to an increase in chlorophyll b concentrations in maize plants subjected to saline water treatment. Biochar is generally considered an efficient method for alleviating the adverse effects of salinity. To enhance plant growth and development while mitigating salinity-induced toxicity, the application of biochar in saline soils must be implemented appropriately.
Keywords: Acacia; Antioxidant activities; Biochar; Corn; Growth; Physiology; Salinity; Soil.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study does not include human or animal subjects. Permission: Permissions or licenses were obtained to collect maize variety (SILVER-2019) and Acacia bark taken from (Regional Agricultural Research Institute Bahawalpur) before starting the research. Statement on guidelines: All experimental studies and experimental materials involved in this research are in full compliance with relevant institutional, national and international guidelines and legislation.
Figures




References
-
- Irin, I. J. & Hasanuzzaman, M. Organic amendments: Enhancing plant tolerance to salinity and metal stress for improved agricultural productivity. Stresses4(1), 185–209 (2024). - DOI
-
- Mahmood, S. et al. Silicon and biochar synergistically stimulate nutrients uptake, photosynthetic pigments, gaseous exchange and oxidative defense to improve maize growth under salinity. Water Air Soil Pollut.235(6), 413 (2024). - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

