Biocompatible ionized air alleviates rat osteoarthritis by modulating polarization from M1 to M2 macrophages
- PMID: 39738316
- PMCID: PMC11685818
- DOI: 10.1038/s41598-024-83198-6
Biocompatible ionized air alleviates rat osteoarthritis by modulating polarization from M1 to M2 macrophages
Abstract
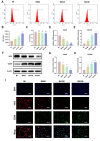
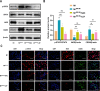
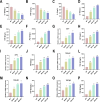
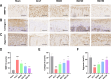
The imbalance in the proportion of M1/M2 macrophage polarization is a crucial contributor to the persistent progression of osteoarthritis (OA). This study aimed to evaluate the effects of low-dose biocompatible ionized air (BIA) on macrophage polarization and its subsequent chondroprotective effects, thereby validating the potential of BIA in slowing the progression of OA. In vitro experiments demonstrated that BIA modulates the polarization of M1 macrophages toward the M2 phenotype via the ROS-mediated STAT6 pathway. This shift reduces the expression of pro-inflammatory mediators while increasing the expression of anti-inflammatory mediators and pro-chondrogenic factors, leading to an improved microenvironment surrounding chondrocytes. The direct benefits of this improved microenvironment include enhanced chondrocyte viability, inhibition of apoptosis, and reduced degradation of the extracellular matrix. In vivo studies in rats showed that BIA inhibited M1 macrophage infiltration in the synovium, upregulated the proportion of M2 macrophages, alleviated cartilage degeneration, and delayed OA progression. This gas-based regulatory strategy may open new avenues for the treatment of OA.
Keywords: Biocompatible ionized air; Macrophages; Osteoarthritis.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no conflicts of interest.
Figures




Similar articles
-
S-propargyl-cysteine attenuates temporomandibular joint osteoarthritis by regulating macrophage polarization via Inhibition of JAK/STAT signaling.Mol Med. 2025 Apr 7;31(1):128. doi: 10.1186/s10020-025-01186-6. Mol Med. 2025. PMID: 40197110 Free PMC article.
-
Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages.Free Radic Biol Med. 2019 Dec;145:146-160. doi: 10.1016/j.freeradbiomed.2019.09.024. Epub 2019 Sep 21. Free Radic Biol Med. 2019. PMID: 31550528
-
Exosomes derived from miR-146a-overexpressing fibroblast-like synoviocytes in cartilage degradation and macrophage M1 polarization: a novel protective agent for osteoarthritis?Front Immunol. 2024 May 23;15:1361606. doi: 10.3389/fimmu.2024.1361606. eCollection 2024. Front Immunol. 2024. PMID: 38846937 Free PMC article.
-
Development of a macrophage polarization-modulating therapeutic agent for osteoarthritis treatment.J Orthop Surg Res. 2025 Mar 14;20(1):279. doi: 10.1186/s13018-025-05679-2. J Orthop Surg Res. 2025. PMID: 40082923 Free PMC article. Review.
-
Macrophage: A Potential Target on Cartilage Regeneration.Front Immunol. 2020 Feb 11;11:111. doi: 10.3389/fimmu.2020.00111. eCollection 2020. Front Immunol. 2020. PMID: 32117263 Free PMC article. Review.
References
-
- Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet393, 1745–1759. 10.1016/s0140-6736(19)30417-9 (2019). - PubMed
-
- Hu, Y. et al. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med.145, 146–160. 10.1016/j.freeradbiomed.2019.09.024 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

