Predicting CTCF cell type active binding sites in human genome
- PMID: 39738353
- PMCID: PMC11686126
- DOI: 10.1038/s41598-024-82238-5
Predicting CTCF cell type active binding sites in human genome
Abstract
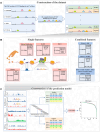
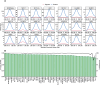
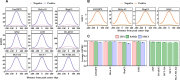
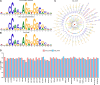
The CCCTC-binding factor (CTCF) is pivotal in orchestrating diverse biological functions across the human genome, yet the mechanisms driving its cell type-active DNA binding affinity remain underexplored. Here, we collected ChIP-seq data from 67 cell lines in ENCODE, constructed a unique dataset of cell type-active CTCF binding sites (CBS), and trained convolutional neural networks (CNN) to dissect the patterns of CTCF binding activity. Our analysis reveals that transcription factors RAD21/SMC3 and chromatin accessibility are more predictive compared to sequence motifs and histone modifications. Integrating them together achieved AUPRC values consistently above 0.868, highlighting their utility in deciphering CTCF transcription factor binding dynamics. This study provides a deeper understanding of the regulatory functions of CTCF via machine learning framework.
Keywords: CTCF binding site; Chromatin accessibility; Convolutional neural networks; RAD21; SMC3.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
CTCF mediates chromatin looping via N-terminal domain-dependent cohesin retention.Proc Natl Acad Sci U S A. 2020 Jan 28;117(4):2020-2031. doi: 10.1073/pnas.1911708117. Epub 2020 Jan 14. Proc Natl Acad Sci U S A. 2020. PMID: 31937660 Free PMC article.
-
DNA Methylation Regulates Alternative Polyadenylation via CTCF and the Cohesin Complex.Mol Cell. 2020 May 21;78(4):752-764.e6. doi: 10.1016/j.molcel.2020.03.024. Epub 2020 Apr 24. Mol Cell. 2020. PMID: 32333838 Free PMC article.
-
Variable Extent of Lineage-Specificity and Developmental Stage-Specificity of Cohesin and CCCTC-Binding Factor Binding Within the Immunoglobulin and T Cell Receptor Loci.Front Immunol. 2018 Mar 8;9:425. doi: 10.3389/fimmu.2018.00425. eCollection 2018. Front Immunol. 2018. PMID: 29593713 Free PMC article.
-
CTCF: a Swiss-army knife for genome organization and transcription regulation.Essays Biochem. 2019 Apr 23;63(1):157-165. doi: 10.1042/EBC20180069. Print 2019 Apr 23. Essays Biochem. 2019. PMID: 30940740 Review.
-
Genome-wide studies of CCCTC-binding factor (CTCF) and cohesin provide insight into chromatin structure and regulation.J Biol Chem. 2012 Sep 7;287(37):30906-13. doi: 10.1074/jbc.R111.324962. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952237 Free PMC article. Review.
References
-
- Vostrov, A. A. & Quitschke, W. W. The zinc finger protein CTCF binds to the APBbeta domain of the amyloid beta-protein precursor promoter Evidence for a role in transcriptional activation. J. Biol. Chem.272, 33353–33359. 10.1074/jbc.272.52.33353 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

