Diagnoses of postpartum urinary retention using next-generation non-piezo ultrasound technology: assessing the accuracy and benefits
- PMID: 39738356
- PMCID: PMC11685570
- DOI: 10.1038/s41598-024-83160-6
Diagnoses of postpartum urinary retention using next-generation non-piezo ultrasound technology: assessing the accuracy and benefits
Abstract
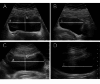
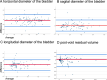
Postpartum urinary retention has a wide range of publicized incidences, likely caused by frequent misdiagnosis of this puerperal complication. Especially covert postpartum urinary retention has a high number of missed diagnoses due to the lack of symptoms and the time-extensive diagnostics via ultrasound, leading to no treatment and no appropriate follow-up. To simplify the diagnosis and establish a screening tool we analyzed the application of portable handheld-ultrasound devices (PUD) as used in Point-of-care diagnostics in comparison to established standard ultrasound devices (SUD). This prospective study aimed to evaluate the reliability of non-piezo, chip-based PUD in comparison to the measurement withSUD, containing a piezo transducer, as golden standard for the ultrasound diagnosis of postpartum urinary retention. Randomly, 100 participants between the first and seventh day after delivery in an obstetric ward underwent ultrasound examinations using a EPIQ 5 W (Philips) as SUD and a Butterfly iQ (Butterfly Network) as PUD to compare the accuracy in bladder size after micturition and the estimated post-void residual volume. Intraclass correlation coefficients, Bland-Altman plots, and Pearson correlation coefficients were used for analyzing the reliability and agreement between the measurements of these devices and were calculated for subgroups as body mass index, mode of delivery and timepoint of delivery. The results show a near-perfect agreement (0.994) and correlation (r = 0.982) for estimated post-void residual volume and for most measurements between the two types of ultrasound devices. The agreement rate for the diagnosis of covert postpartum urinary retention is 100%. Subgroup analyses lack a significant difference reflected by agreement and correlation rates. These findings affirm the high reliability of PUD for the diagnosis of postpartum urinary retention and supports their integration into daily clinical practice, thereby simplifying regular controls of the bladder by physicians during daily rounds on the ward. This technology may allow a higher diagnosis rate so that patient care can be optimized and the long-term impact on continence and quality of life can be studied and analysed.
Keywords: Chip-based; Obstetrics; POCUS; Personal-device-based-point-of-care-ultrasound; Piezo-based; Point-of-care-ultrasound; Postpartum; Semiconductors; Urinary retention; Urology.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

