Pharmacokinetics of tebipenem pivoxil used in children suffering from shigellosis: a pilot study in Bangladesh
- PMID: 39738375
- PMCID: PMC11685405
- DOI: 10.1038/s41598-024-83549-3
Pharmacokinetics of tebipenem pivoxil used in children suffering from shigellosis: a pilot study in Bangladesh
Abstract
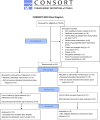
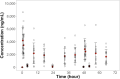
With increasing antibiotic resistance in gram-negative bacteria, including those causing Shigellosis, evidence of safety and pharmacokinetics data on new oral antibiotics is crucial. We aimed to investigate the safety and pharmacokinetic properties of an oral carbapenem, tebipenem pivoxil, along with it's ability to produce desired results in childhood shigellosis. This randomized pilot clinical trial was conducted at Dhaka Hospital, icddr,b in 2022 between May and September. Thirty suspected shigellosis cases aged 24-59 months were randomized across two treatment groups equally: tebipenem pivoxil and azithromycin. Pharmacokinetics of tebipenem was assessed among fifteen children who received tebipenem pivoxil using Noncompartmental analysis (NCA). Clinical (absence of fever, abdominal pain/tenderness, diarrhoea, blood in stool, or death before Day-3) and microbiological (absence of Shigella on Day-7 culture) success after the antibiotic interventions were also evaluated. Sociodemographic and clinical characteristics were comparable between the randomization arms. Twelve children, each in the azithromycin arm and tebipenem arm, were positive for Shigella by culture on enrolment. Cmax values of 5053.3, 2546.0, and 3759.2 ng/mL were observed for plasma tebipenem on Day-0, 1, and 2 respectively. Clinical success was observed among seven participants in each arm while two in the azithromycin arm and three in the tebipenem arm failed microbiologically. The tolerability and efficaciousness of tebipenem pivoxil appear to be comparable to azithromycin in treating childhood shigellosis in Bangladesh. We recommend a larger clinical trial to determine non-inferiority of tebipenem in regards to the current treatment guidelines.
Keywords: Dysentery; Pharmacokinetics; Shigellosis; Tebipenem; Under-5 children.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: AN is an employee of the GSK group of companies and owns shares in it. AN declares no other financial and non-financial relationships and activities. The other authors declare that they have no competing interests. Ethics declaration: The randomized controlled trial was registered at ClinicalTrials.gov (NCT05121974, 16/11/2021) and the research protocol was approved by the Institutional Review Board (IRB) of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) (approval number: PR-21005). All clinical aspects of the study were supervised by the investigators and study physicians. Names of the participants were de-identified prior to analysis. Written informed consent was collected from each participant prior to enrolment. The trial was carried out in full compliance with good clinical practices guidelines.
Figures
References
-
- WHO. Diarrhoea, <https://www.who.int/health-topics/diarrhoea> (2023).
-
- UNICEF. Diarrhoea, <https://data.unicef.org/topic/child-health/diarrhoeal-disease/> (2023).
-
- Exchange, I. G. H. D. Global Burden of Disease Study 2019, <https://vizhub.healthdata.org/gbd-results/> (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical