Arctiin alleviates knee osteoarthritis by suppressing chondrocyte oxidative stress induced by accumulated iron via AKT/NRF2/HO-1 signaling pathway
- PMID: 39738432
- PMCID: PMC11685860
- DOI: 10.1038/s41598-024-83383-7
Arctiin alleviates knee osteoarthritis by suppressing chondrocyte oxidative stress induced by accumulated iron via AKT/NRF2/HO-1 signaling pathway
Abstract
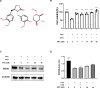
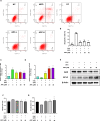
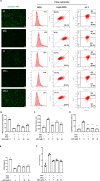
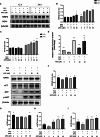
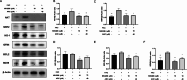
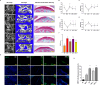
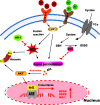
Iron overload (IO) was considered to be a risk factor for cartilage degradation in knee osteoarthritis (KOA) advancement. However, few drugs were found to improve cartilage degeneration by alleviating multiple cell death induced by the impaired iron level of the knee joints. We aimed to elucidate that Arctiin (ARC) plays a role in managing KOA caused by accumulated iron levels by restoring chondrocyte apoptosis and ferroptosis. Single-cell RNA sequencing analysis was used to discover the disparities in chondrocytes between KOA patients and non-KOA individuals. CCK-8 assay was performed to detect chondrocyte viability. Annexin V-FITC/PI staining determined the cell apoptosis rate. The fluorescence density reflected the iron content, ROS, lipid-ROS, and mitochondrial membrane potential. Q-RTPCR and Western Blotting were used to detect the expression levels of genes and proteins expression. Micro-CT and Safranin O-Fast Green staining were used to detect the phenotype of the knee joints. ARC increased cell viability and inhibited chondrocyte apoptosis. Further, ARC acts as an anti-ferroptosis effect by reducing the intracellular iron, ROS, and lipid-ROS content and restoring mitochondrial damage. Based on the results of scRNA-seq, we found that ARC can play a role by activating AKT/NRF2/HO-1 signaling pathway. In vivo, ARC can significantly improve the severity of KOA caused by IO. ARC alleviates oxidative stress in chondrocytes via the AKT/NRF2/HO-1 signaling pathway, suggesting the potential application of ARC in KOA.
Keywords: Apoptosis; Cartilage degeneration; Ferroptosis; Herbal medicine; Iron overload.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Written informed consent for publication was obtained from all participants. Competing interests: The authors declare no competing interests. Institutional Review Board Statement: All animal experiments were approved by the Review Board of The First Affiliated Hospital of Guangzhou University of Chinese Medicine (Ethic NO. TCMF1-2021029).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

