PPAR-γ agonist mitigates intestinal barrier dysfunction and inflammation induced by Clostridioides difficile SlpA in vitro
- PMID: 39738433
- PMCID: PMC11686163
- DOI: 10.1038/s41598-024-83815-4
PPAR-γ agonist mitigates intestinal barrier dysfunction and inflammation induced by Clostridioides difficile SlpA in vitro
Abstract
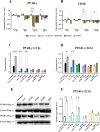
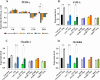
Clostridioides difficile is the leading cause of healthcare- and antibiotic-associated diarrhea. Surface layer protein A (SlpA), an essential component of the bacterium's outermost layer, contributes to colonization and inflammation. The peroxisome proliferator-activated receptor gamma (PPAR-γ) has been demonstrated to improve intestinal integrity and prevent inflammation in host cells. Here, we investigated the role of PPAR-γ in SlpA-mediated inflammation in Caco-2 cells and THP-1 derived macrophages. The extraction of SlpA was carried out for three toxigenic C. difficile clinical strains (RT126, RT001, RT084) and a non-toxigenic strain (ATCC 700057). The gene expression of tight junction (TJ) proteins and inflammatory markers was determined using RT-qPCR. The production of proinflammatory cytokines and nitric oxide was measured by ELISA and Griss reaction, respectively. Western blotting was performed to detect PPAR-γ level before and after adding its agonist, pioglitazone. SlpA of C. difficile strains enhanced the expression of TLR-4, NF-κB, MyD88, IL-17, MCP-1, IL-8, IL-6, TNF-α, IL-1β, whilst the gene expression level of JAM-A, claudin-1, occludin, PPAR-γ and its receptor (CD36) was decreased in both Caco-2 cells and THP-1 derived macrophages. Moreover, pioglitazone caused a notable elevation in the expression level of PPAR-γ, only following treatment with RT126 SlpA. Besides, pioglitazone pretreatment improved TJ impairment in Caco-2 cells and attenuated proinflammatory cytokine expression in both SlpA-treated cell lines. SlpA can attenuate PPAR-γ expression, trigger TJ disruption, and stimulate inflammatory response in host cells. Notably, these events could be reversed by pretreatment of cells with PPAR-γ agonist. Further experiments are required to corroborate the present findings.
Keywords: Clostridioides difficile; Caco-2 cells; Peroxisome proliferator-activated receptor gamma; Pioglitazone; Surface layer protein A; THP-1 derived macrophages.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

