Understanding the synergistic interaction between a 1,3,4-thiadiazole derivative and amphotericin B using spectroscopic and theoretical studies
- PMID: 39738538
- PMCID: PMC11686287
- DOI: 10.1038/s41598-024-83180-2
Understanding the synergistic interaction between a 1,3,4-thiadiazole derivative and amphotericin B using spectroscopic and theoretical studies
Abstract
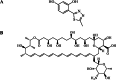
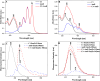
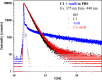
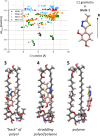
We present a comprehensive spectroscopic study supported by theoretical quantum chemical calculations conducted on a molecular system (4-(5-methyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol (C1) and the antibiotic Amphotericin B (AmB)) that exhibits highly synergistic properties. We previously reported the strong synergism of this molecular system and now wish to present related stationary measurements of UV-Vis absorption, fluorescence, and fluorescence anisotropy in a polar, aprotic solvent (DMSO and a PBS buffer), followed by time-resolved fluorescence intensity and anisotropy decay studies using different ratios of the selected 1,3,4-thiadiazole derivative to Amphotericin B. Absorption spectra measured for the system revealed discrepancies in terms of the shapes of absorption bands, particularly in PBS. Fluorescence emission spectra revealed that the addition of C1 molecules triggered significant changes in the emission spectra of the system. Measurements of the fluorescence lifetimes and fluorescence anisotropy supported by synchronous spectra clearly showed evidence of disaggregation. The AmB molecular aggregates indicated interaction of C1 with the antibiotic at points responsible for the formation of dimer structures. The spectroscopic results were further corroborated, analyzed, and interpreted using the methods of quantum mechanical modelling. Analyses based on the density functional tight-binding and time-dependent density functional theory confirmed that molecular interactions between "small" molecules and AmB lead to a significant increase in the clinical efficacy of the antibiotic.
Keywords: 1,3,4-thiadiazole (C1); [TD]DFT; Amphotericin B (AmB); DFT; Molecular spectroscopy; Synergism.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Halde, C., Wright, E. T., Pollard Jr, W. H., Newcomer, V. D. & Sternberg, T. H. The effect of amphotericin B upon the yeast flora of the gastrointestinal tract of man. (1957). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

