Efficacy and safety of single and double catheter intrathecal drug delivery systems in patients with refractory neck and abdominal cancer pain
- PMID: 39738565
- PMCID: PMC11686266
- DOI: 10.1038/s41598-024-83799-1
Efficacy and safety of single and double catheter intrathecal drug delivery systems in patients with refractory neck and abdominal cancer pain
Abstract
Intrathecal drug delivery systems (IDDS) is a crucial for treating refractory cancer pain, but their effectiveness in patients with pain across multiple spinal segments is limited by the localized spread of pain relief medication. Our team innovatively implanted double-catheter IDDS to manage pain related to neck and abdominal cancer. While this may represent a new solution, the efficacy, safety, and cost-effectiveness remain unclear. A multi-center retrospective cohort study. Pain management and medical oncology departments of six hospitals in various regions of China. 62 patients with neck or abdominal cancer pain were enrolled from November 2019 to June 2024. Patients were divided into two groups: the double-catheter IDDS group (n = 26) and the single-catheter IDDS groups (n = 36). Propensity score matching was employed to create a balanced cohort of 48 patients. The primary outcome was pain control, assessed using Numeric Rating Scale [NRS]), breakthrough pain (BTP), and opioid consumption, including intrathecal morphine dose [IDMED] and oral daily morphine dose [ODMED]. No significant differences were observed in the NRS score and IDMED between the double-catheter and single-catheter groups prior to surgery, one day post-surgery, and at hospital discharge (p > 0.05). However, one-month post-surgery, the NRS score was significantly lower in the double-catheter group compared to the single-catheter group, while the IDMED was significantly higher compared to the single-catheter group (p < 0.05). A significantly higher number of BTP episodes and ODMED was observed in the single-catheter group compared to the two-catheter group at one day post-surgery, at hospital discharge, and one-month post-surgery (p < 0.05). The duration of hospitalization and the incidence of adverse events did not differ significantly between the two groups (p > 0.05). However, the cumulative hospitalization expenses, IDDS opioid costs per month, and refill costs calculated for a month were significantly higher in the double-catheter group compared to the single-catheter group. Conversely, the monthly oral opioid costs and total costs of analgesic were significantly lower in comparison to the single-catheter group (p < 0.05). The preliminary findings of this study, both single- and double-catheter IDDS effectively manage cancer pain in neck and abdominal cancer patients without increasing adverse events. Despite higher initial costs, double-catheter IDDS demonstrates superior long-term pain control, a reduced incidence of BTP, and lower overall monthly analgesic costs. However, due to the limitations of the study's sample size and patient survival time, further research is needed to assess the long-term benefits.
Keywords: Comparative analysis; Double-catheter; IDDS; Refractory cancer pain; Single-catheter.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All included patients gave their oral and written informed consent. The study was approved by the Ethics Committee (full name: the Ethics Committee of Sichuan Provincial Cancer Hospital) (reference number SCCHEC-03-2024-071) to the Department of Anesthesiology, Sichuan Cancer Hospital & Institute.
Figures
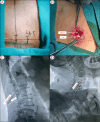
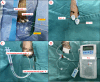
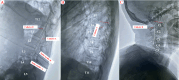

References
-
- Hindle, A. Intrathecal opioids in the management of acute postoperative pain. Continuing Educ. Anaesth. Crit. Care Pain. 8 (3), 81–85 (2008). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

