Biophysics and electrophysiology of pulsed field ablation in normal and infarcted porcine cardiac ventricular tissue
- PMID: 39738639
- PMCID: PMC11686391
- DOI: 10.1038/s41598-024-83683-y
Biophysics and electrophysiology of pulsed field ablation in normal and infarcted porcine cardiac ventricular tissue
Abstract
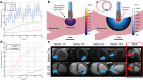
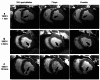
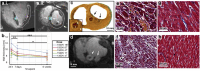
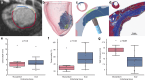
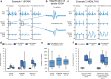
Pulsed Field Ablation (PFA) is a new ablation method being rapidly adopted for treatment of atrial fibrillation, which shows advantages in safety and efficiency over radiofrequency and cryo-ablation. In this study, we used an in vivo swine model (10 healthy and 5 with chronic myocardial infarct) for ventricular PFA, collecting intracardiac electrograms, electro-anatomical maps, native T1-weighted and late gadolinium enhancement MRI, gross pathology, and histology. We used 1000-1500 V pulses, with 1-16 pulse trains to vary PFA dose. Lesions were assessed at 24 h, 7 days, and 6 weeks in healthy and at 48 h in infarcted ventricles. Comparisons of lesion sizes using a numerical model enabled us to determine lethal electric field thresholds for cardiac tissue and its dependence on the number of pulse trains. Similar thresholds were found in normal and infarcted hearts. Numerical modeling and temperature-sensitive MRI confirmed the nonthermal nature of PFA, with less than 2% of a lesion's volume at the highest dose used being attributed to thermal damage. Longitudinal cardiac MRI and histology provide a comprehensive description of lesion maturation. Lesions shrink between 24 h and 7 days post-ablation and then remain stable out to 6 weeks post-ablation. Periprocedural electrograms analysis yields good correlation with lesion durability and size.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Competing interests: G Wright receives research support related to this work from Medtronic, GE Healthcare, and ADAS 3D, D Miklavcic receives research grants from Medtronic and is retained consultant of Medtronic, B Kos is a consultant of Medtronic, A Verma receives research grants from Medtronic, Biosense Webster, Bayer, and serves in advisory capacity to Medtronic, Biosense Webster, Abbott, Adagio Medical, and Medlumics.All the remaining authors declare no conflict of interest.
Figures



References
-
- Reddy, V. Y. et al. Pulsed field or conventional thermal ablation for Paroxysmal Atrial Fibrillation. N Engl. J. Med.389, 1660–1671 (2023). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

