Preliminary study of utilizing a patient derived tumor spheroid model to augment precision therapy in metastatic brain tumors
- PMID: 39738713
- PMCID: PMC11685586
- DOI: 10.1038/s41598-024-83409-0
Preliminary study of utilizing a patient derived tumor spheroid model to augment precision therapy in metastatic brain tumors
Abstract
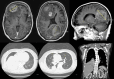
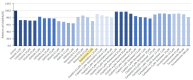
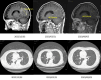
Treating metastatic brain tumors remains a significant challenge. This study introduces and applies the Patient-Derived Tumor Spheroid (PDTS) system, an ex vivo model for precision drug testing on metastatic brain tumor. The PDTS system utilizes a decellularized extracellular matrix (dECM) derived from adipose tissue, combined with the tumor cells, to form tumor spheroids. These spheroids were subsequently used to test anticancer drugs, with results compared to the clinical outcomes observed after administering these treatments to patients. To assess the validity of the data, the correlation between the drug responses observed in the PDTS model and actual patient outcomes was analyzed. Chi-square tests evaluated the significance of associations between lab predictions and clinical outcomes, using a significance threshold of p < 0.05. In preliminary data, 17 patients met the criteria for final analysis, which showed an overall 57% accuracy (p-value = 0.463), with improvements to 73% accuracy (p-value = 0.072) when patients receiving certain treatments were excluded. This PDTS offers real-time results within three weeks, simultaneous testing of multiple drugs, and the ability to culture and store tumor cells for reproducibility. Despite some limitations, further development of this model could enhance its clinical application and improve patient outcomes.
Keywords: Metastatic brain tumor; Micro-organosphere; Patient-derived organoid; Patient-derived tumor spheroids; Precision oncology.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
A Review on Patient-derived 3D Micro Cancer Approach for Drug Screen in Personalized Cancer Medicine.Curr Cancer Drug Targets. 2025;25(2):118-130. doi: 10.2174/0115680096285910240206044830. Curr Cancer Drug Targets. 2025. PMID: 38445692 Review.
-
Complex Tumor Spheroids, a Tissue-Mimicking Tumor Model, for Drug Discovery and Precision Medicine.SLAS Discov. 2021 Dec;26(10):1298-1314. doi: 10.1177/24725552211038362. Epub 2021 Nov 12. SLAS Discov. 2021. PMID: 34772287
-
3D stem-like spheroids-on-a-chip for personalized combinatorial drug testing in oral cancer.J Nanobiotechnology. 2024 Jun 18;22(1):344. doi: 10.1186/s12951-024-02625-y. J Nanobiotechnology. 2024. PMID: 38890730 Free PMC article.
-
Intertumoral heterogeneity in patient-specific drug sensitivities in treatment-naïve glioblastoma.BMC Cancer. 2019 Jun 25;19(1):628. doi: 10.1186/s12885-019-5861-4. BMC Cancer. 2019. PMID: 31238897 Free PMC article.
-
Experimental anti-tumor therapy in 3-D: spheroids--old hat or new challenge?Int J Radiat Biol. 2007 Nov-Dec;83(11-12):849-71. doi: 10.1080/09553000701727531. Int J Radiat Biol. 2007. PMID: 18058370 Review.
Cited by
-
Patient-derived organotypic tumor spheroids, tumoroids, and organoids: advancing immunotherapy using state-of-the-art 3D tumor model systems.Lab Chip. 2025 Jun 24;25(13):3038-3059. doi: 10.1039/d5lc00062a. Lab Chip. 2025. PMID: 40509886 Review.
-
Standing balance test predicts the Berg Balance Scale score in patients with stroke using principal component analysis.Sci Rep. 2025 May 21;15(1):17653. doi: 10.1038/s41598-025-99710-5. Sci Rep. 2025. PMID: 40399582 Free PMC article.
References
-
- Voest, E. E. & Bernards, R. DNA-guided precision medicine for cancer: a case of irrational exuberance? Cancer Discov. 6, 130–132 (2016). - PubMed
-
- Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med.21, 1318–1325 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

