Teknonaturalist: A Snakemake Pipeline for Assessing Fungal Diversity From Plant Genome Bycatch
- PMID: 39739202
- PMCID: PMC11887601
- DOI: 10.1111/1755-0998.14056
Teknonaturalist: A Snakemake Pipeline for Assessing Fungal Diversity From Plant Genome Bycatch
Abstract
Relatively little is known of the host associations and compatibility of fungal plant pathogens and endophytes. Publicly available plant genomic DNA can be mined to detect incidental fungal DNA, but taxonomic assignment can be challenging due to short lengths and variable discriminative power among different genomic regions and taxa. Here, we introduce a computationally lightweight and accessible Snakemake pipeline for rapid detection and classification (identification and assignment to taxonomic rank) of pathogenic and endophytic fungi (and other fungi associated with plants) that targets the internal transcribed spacer (ITS) region, a fungal barcode standard. We include methods for maximising query sequence length, which gives higher support for ITS1 and ITS2 taxonomic classifications by extending to other fragments of the ITS region and providing taxon-specific local cut-off and confidence scores. We demonstrate our pipeline with a case study using public genomic sequence data for six diverse plant species, including four species within Betula, an ecologically and economically important broadleaved forest tree genus, a shrub and a grass. Our pipeline classified fungi within minutes to a few hours per host individual, with 204 different fungal genera identified at high confidence (≥ 70%). Our pipeline detected and classified pathogenic and endophytic genera known to associate with Betula, and many others with no prior record of association. Our pipeline, leveraging existing sequence data, has several potential applications, including detecting cryptic fungal pathogens and helping characterise the endophytic fungal microbiome, bioprospecting commercially useful fungal species, and determining the plant host range of fungi.
Keywords: ITS; endophyte; fungi; genome bycatch; plant pathogens; plant‐fungal associations.
© 2024 The Author(s). Molecular Ecology Resources published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
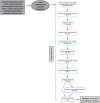
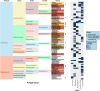
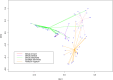
References
-
- Abarenkov, K. , Zirk A., Piirman T., et al. 2022. “UNITE General FASTA Release for Fungi 2.” UNITE Community. 10.15156/BIO/2483912. - DOI
-
- Abarenkov, K. , Zirk A., Piirman T., et al. 2024. “Full UNITE+INSD Dataset for Fungi.” UNITE Community. 10.15156/BIO/2959330. - DOI
-
- Anagnostakis, S. L. 1987. “Chestnut Blight: The Classical Problem of an Introduced Pathogen.” Mycologia 79, no. 1: 23–37.
-
- Andrews, S. 2010. “FastQC a Quality‐Control Tool for High‐Throughput Sequence Data.” [Computer Software]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

